More Information
Submitted: April 01, 2023 | Approved: April 13, 2023 | Published: April 14, 2023
How to cite this article: Kanoun RY, Abdeljelil NB, Mekni S, Kasdallah M, Ouerghi R, et al. Role of measurable residual disease quantified by 4 to 6 color flow cytometry before allogeneic hematopoietic stem cell transplantation for high-risk Philadelphia-negative acute lymphoblastic leukemia. J Stem Cell Ther Transplant. 2023; 7: 016-023
DOI: 10.29328/journal.jsctt.1001031
Copyright License: © 2023 Al-Anazi KA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Acute lymphoblastic leukemia; Measurable residual disease; Multicolor flow cytometry; Allogeneic hematopoietic stem cell transplantation; Myeloablative conditioning regimen
Role of measurable residual disease quantified by 4 to 6 color flow cytometry before allogeneic hematopoietic stem cell transplantation for high-risk Philadelphia-negative acute lymphoblastic leukemia
Rimmel Yosra Kanoun1*, Nour Ben Abdeljelil1, Sabrine Mekni1, Manel Kasdallah2, Rihab Ouerghi1, Insaf Ben Yaiche1, Lamia Torjemane1, Dorra Belloumi1, Ines Turki1, Ines Safra2, Saloua Ladeb1 and Tarek Ben Othman1
1National Bone Marrow Transplantation Center, Department of Hematology and Transplant, Tunis, Tunisia
2Pasteur Tunis Institute, Central Laboratory of Hematology, Tunis, Tunisia
*Address for Correspondence: Rimmel Yosra Kanoun, National Bone Marrow Transplantation Center, Department of Hematology and Transplant, Tunis, Tunisia, Email: yousr2008@hotmail.fr; kanounrimmelyosra@gmail.com
Background: Measurable residual disease (MRD) status before allogeneic hematopoietic stem cell transplantation (AHSCT) is commonly associated with a high risk of relapse. It is still uncertain whether AHSCT could overcome the negative impact of MRD positivity (MRD+), especially in patients with high-risk Philadelphia negative acute lymphoblastic leukemia (Ph-negative ALL).
Materials and methods: An observational retrospective study was conducted on patients with high-risk Ph-negative ALL who underwent AHSCT between January 2005 and June 2022. The patients selected were in complete remission (CR): with 80% in CR1 (n = 69) and 20% in CR2 (n = 17). Graft sources were bone marrow (BM) in 71% of patients and peripheral blood stem cells in 29% of patients. The conditioning regimen was TBI or chemotherapy-based (CT). Bone marrow MRD level was quantified using 4-6 color multiparametric flow cytometry (MFC). The threshold for MRD positivity was ≥ 0.1%.
Results: The study included 86 patients (45 B-ALL and 41 T-ALL) with a median age of 18 years (range, 4–55 years). The median level of MRD pre-AHSCT (pre-MRD) was 0.4×10-3 (range, 0.01-75.6×10-3). After a median follow-up of 25 months (range 1-205 months), the cumulative incidence of relapse (CIR) was significantly higher in the MRD+ group (39% vs. 20%, p = 0.04). The median time of relapse post-AHSCT was 14 months (range, 1-203 months) in the MRD+ group and 32 months (range, 4-209 months) in the MRD- group (p = 0.28). Non-relapse mortality (NRM) was 15% in both groups (p = 0.97). The 2-year estimated overall survival (OS) and event-free survival (EFS) were 61% vs. 74% (p = 0.07) and 58% vs. 70% (p = 0.10) in the MRD+ and MRD- groups, respectively. A subgroup analysis in MRD+ patients showed that a TBI-based conditioning regimen was distinctly associated with lower CIR (22% vs. 60% respectively, p = 0.04), improved OS (82% vs. 36% respectively, p = 0.007) and better EFS (73% vs. 38%, p = 0.04) compared to CT-based. In a multivariate analysis, pre-AHSCT MRD+ status and non-TBI-based conditioning were significantly associated with inferior OS (OR, 2.30; 95% CI, [1.027-5.168], p = 0.04 and OR, 3.91; 95% CI, [1.624-9.418], p = 0.002, respectively). The only predicting factor of lower EFS was the non-TBI-based regimen (OR, 2.82; 95% CI, [1.308-6.097], p = 0.008). Non-TBI-based and CR2 were significantly associated with higher CIR (OR, 6.25; 95% CI, [1.947-20.055], p = 0.002 and OR, 4.74; 95% CI, [1.197-18.791], p = 0.03, respectively). Peripheral stem cell source was significantly associated with higher NRM (OR, 6.55; 95% CI, [1.488-28.820], p = 0.01).
Conclusion: High-risk Ph-negative ALL patients with MRD ≥ 10-3 prior AHSCT had lower OS compared to MRD- patients and may benefit from TBI as a conditioning regimen before AHSCT.
Outcomes of acute lymphoblastic leukemia (ALL) have improved with the development of pediatric-based protocols and the introduction of immunotherapy [1]. Despite this progress, relapse remains the major cause of treatment failure and death after AHSCT [2]. The measurable residual disease (MRD) status has been validated as an important factor in AHSCT, as several studies have shown significantly inferior outcomes for ALL patients with positive MRD. As a result, MRD has been recently proposed as a tool to guide pre-transplant strategies and post-transplant immunotherapy in order to prevent relapse [3]. The aim of our study is to evaluate the role of MRD quantified by 4-6 color Multiparametric Flow Cytometry (MFC) before AHSCT in patients with high-risk Philadelphia-negative (Ph-negative) ALL.
Patients
A retrospective study of 86 consecutive patients with high-risk Ph-negative ALL in first or second CR (CR1, CR2) diagnosed using the EGIL classification, treated according to EORTC-58951 and GRAALL protocol who underwent AHSCT from HLA-identical sibling-donor, between January 2005 and June 2022. The inclusion criteria were: morphological bone marrow (BM) and cytogenetic CR at the time of AHSCT, defined as less than 5% blasts by morphological examination, normal karyotype, and the availability of BM aspirates for MRD assessment prior to AHSCT. Patients without assessment of MRD before AHSCT were excluded. High-risk ALL included high white blood count at diagnosis (> 30.000/mm3 for B-ALL and > 100.000/mm3 for T-ALL), cytogenetic abnormalities [t(4,11), monosomal karyotype, t(1,19)], induction failure and MRD+ status prior to AHSCT.
MRD assessment by multiparametric flow cytometry
The MRD assessment was performed on fresh BM aspirate samples. Standard MFC assays were used to detect residual leukemic cells by relying on the assessment of aberrant leukemia-associated immunophenotypes (LAIPs), which constitute either an aberrant expression of myeloid antigens, an increased, decreased density or lack of antigens normally expressed on benign lymphoid cell precursors.
All LAIPs detected on leukemic blasts at the time of diagnosis are assessed and constitute MRD when still detectable in the remission sample. Panels of several antibody combinations according to LAIPs were used for MRD detection recognizing markers of progenitors (CD45 dim, CD34, HLADR, CD38), lymphoid T (cCD3, sCD3, CD2, CD5, CD7, CD4, CD8, CD1a), lymphoid B (CD10, CD19, CD20, CD22), myeloid (CD13, CD33, CD117) and other markers (CD56).
0.5-1 million events per tube were acquired on a Becton Dickinson (BD) FACS Calibur four-color (2005- 2009) and BD FACS Cant II six–color (2009-2022). The significant level of MRD was set up by a logarithmic scale.
Patients were categorized into two groups according to MRD analysis: MRD-positive patients (MRD+) with MRD level ≥ 10-3 and MRD-negative patients (MRD-) with MRD level < 10-3.
Conditioning regimen
All patients received a myeloablative conditioning regimen. Total body irradiation (TBI) was delivered at the dose of 3.3 grays during 3 consecutive days associated with Etoposide 60 mg/kg×1 day or Cyclophosphamide 60 mg/kg×2 days. Non-TBI, myeloablative regimen included TBF regimen with Thiotepa 5 mg/kg×2 days, Bisulvex 3.2 mg/kg×3 days and Fludarabine 50 mg/m2×3 days or Bisulvex-Cyclophosphamide regimen.
Stem cell source
Hematopoietic stem cell transplantation is performed using bone marrow or peripheral blood stem cells from geno-identical sibling donors.
Graft-versus-host disease prophylaxis
Graft-versus-host disease (GVHD) prophylaxis consisted of a combination of methotrexate at the dose of 10 mg/m2 on days 1, 3 and 6 and cyclosporine 1.5 mg/kg/12h since day-1 in patients > 12 years and cyclosporine alone for patients < 12 years.
Statistical analysis
Patient characteristics were compared according to MRD status. For qualitative variables, the χ2 or Fisher’s exact test was used, and to compare continuous variables student test was used. Overall survival (OS) was calculated as the time from AHSCT to death or last contact for those alive. Event-free survival (EFS) was defined as the time from AHSCT to the first event (relapse, death) or the date of last contact for those who were event-free and were performed by the Kaplan-Meier method and compared by the log-rank test. Cumulative incidences of relapse (CIR), non-relapse mortality (NRM), and acute and chronic GVHD were calculated by using competing risks, and comparisons were made using the Gray test. The competing risk for relapse is NRM and the competing risk for NRM is relapse. Acute GVHD and chronic GVHD were defined according to Glucksberg, et al. and Shulman, et al. respectively [4,5]. Variables with p < 0.2 were included in a Cox proportional hazards model with time-dependent variables. p - values < 0.05 were considered statistically significant for all comparisons.
Patients’ characteristics
Eighty-six patients with high-risk Ph-negative ALL were included in this study. Table 1 summarizes the characteristics of these patients. The characteristics of our population were fairly homogeneous. However, a longer median follow-up duration was observed in the MRD- group. There was a higher proportion of patients with high cytogenetic risk in the MRD+ group, and more PBSC transplants in the MRD- group.
| Table 1: Patients’ characteristics. | |||
| MRD negative (< 10-3) | MRD positive (≥ 10-3) | p | |
| N | 44 (51%) | 42 (49%) | |
| The median age in years (range) | 17 (5-48) | 18 (4-55) | 0.84 |
| Age < 18 years ≥ 18 years |
22 (50%) 22 (50%) |
20 (48%) 22 (52%) |
0.82 |
| Patient sex Male Female |
26 (59%) 18 (41%) |
32 (76%) 10 (24%) |
0.09 |
| ALL subtype B-ALL T-ALL |
23 (52%) 21 (48%) |
22 (52%) 20 (48%) |
0.99 |
| WBC at diagnosis B-ALL, ≥30.000/mm3 T-ALL, ≥100.000/mm3 |
10 (23%) 6 (14%) |
6 (14%) 7 (17%) |
|
| Cytogenetic risk Standard-risk High-risk unknown |
29 (66%) 6 (14%) 10 (23%) |
24 (57%) 11 (26%) 8 (19%) |
0.45 |
| Post-induction evaluation (65 evaluable) MRD < 0.1% MRD ≥ 0.1% Failure |
20 (45%) 16 (36%) 11 (25%) |
15 (36%) 14 (33%) 13 (31%) |
0.76 |
| Remission status at AHSCT CR1 CR2 |
33 (75%) 11 (25%) |
36 (86%) 6 (14%) |
0.21 |
| Median time diagnosis-AHSCT in months (range) |
8.5 (4-135) | 7 (4-63) | 0.65 |
| Sex mismatch (Donor/Recipient) Female to male Others |
12 (27%) 32 (73%) |
18 (43%) 24 (57%) |
0.13 |
| Conditioning regimen TBI-based CT-based |
24 (55%) 20 (45%) |
23 (55%) 19 (45%) |
0.98 |
| Stem cell source BM PBSC |
28 (64%) 16 (36%) |
33 (79%) 9 (21%) |
0.13 |
| The median number of stem cells infused Median CMN *108/kg (range) Median CD34 *106/kg (range) |
2.2 (0.88-5.8) 4.8 (3.63-6.82) |
2.4 (1.15-4.9) 4.27 (1.07-6.36) |
|
| GVHD prophylaxis Cyclosporine+MTX Cyclosporine |
29 (66%) 15 (34%) |
31 (74%) 11 (26%) |
0.49 |
| Median follow-up in months (range) | 32 (5-205) | 15 (1-203) | 0.13 |
| ALL: Acute Lymphoblastic Leukemia; AHSCT: Allogeneic Hematopoietic Stem Cell Transplantation; WBC: White Blood Cells; CR: COMPLETE REMISSION; CR1: First Complete Remission; CR2: Second Complete Remission; CT: Chemotherapy; TBI: Total Body Irradiation; BM: Bone Marrow; PBSC: Peripheral Blood Stem Cells; MRD: Minimal Residual Disease; CMN: Mononuclear Cells; GVHD: Graft-Versus-Host Disease. | |||
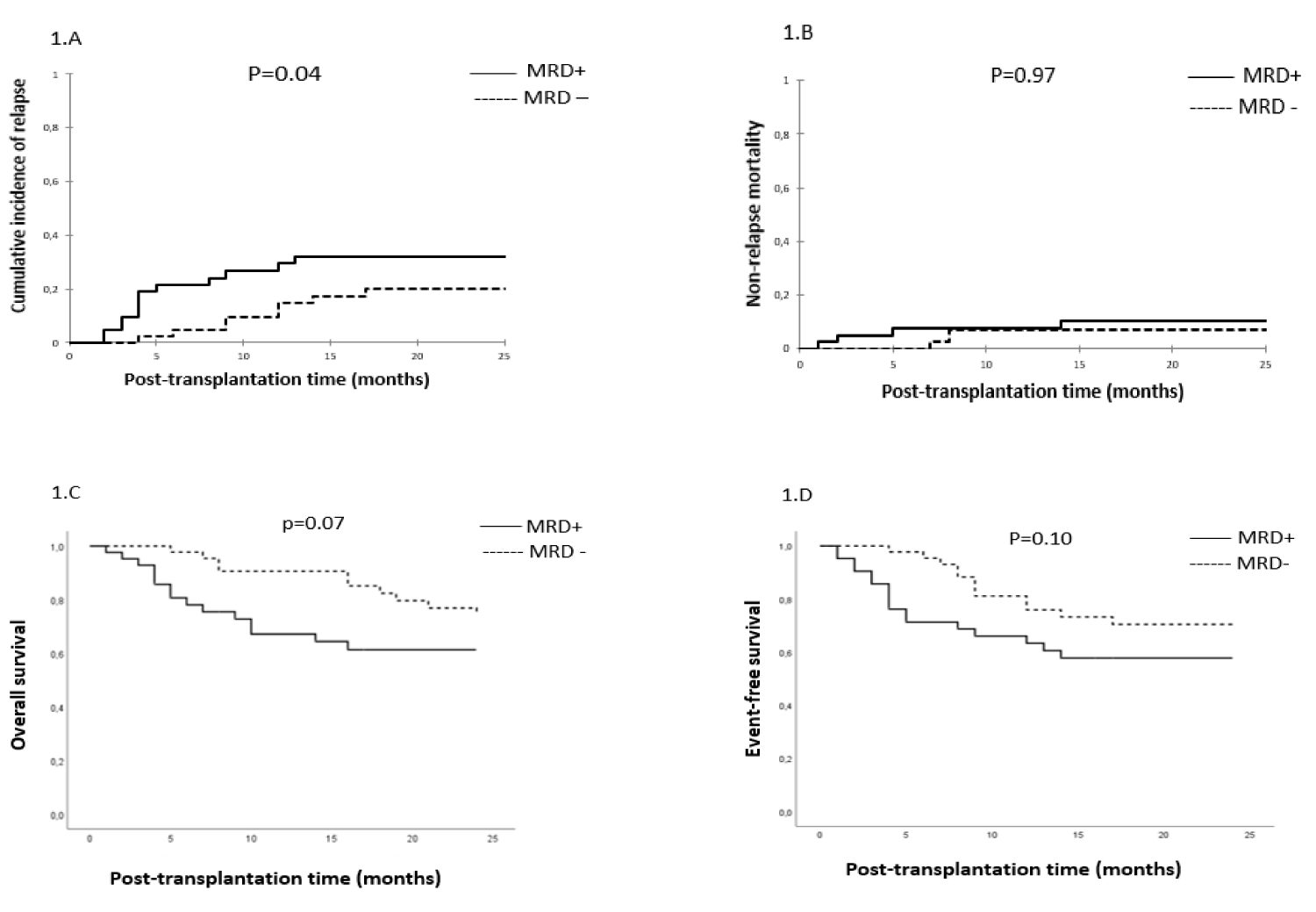
Impact of MRD prior to transplantation on survival, cumulative incidences of relapse and NRM: After a median follow-up of 25 months (range, 1-205 months), the CIR, CI of NRM, the 2-year OS and EFS were 30%, 15%, 68% and 64%, respectively in the entire cohort.
We didn’t find a significant impact of MRD at the threshold of 10-4 (data not shown), the results of our study were compared to the threshold of 10-3.
Relapse: In univariate analysis, the CIR was significantly higher in patients with MRD+ compared to MRD- patients (39% vs. 20% respectively, p = 0.04) (Figure 1A). The median time to relapse was 14 months (range, 1-203 months) in the MRD+ group and 32 months (range, 4-209 months) in MRD– group (p = 0.28). In a multivariate analysis, non-TBI-based regimen and CR2 were significantly associated with higher CIR (OR, 6.25; 95% CI, [1.947-20.055], p = 002 and OR, 4.74; 95% CI, [1.197-18.791], p = 0.03, respectively). A trend towards a significantly higher CIR was observed with MRD+ status (OR, 2.92; 95% CI, [0.937-9.079], p = 0.06). PBSC was associated with a lower CIR with a trend towards significance (OR, 0.26; 95% CI, [0.064-1.035], p = 0.05).
NRM: The CI incidence of NRM was similar in both groups (15% vs. 15%, p = 0.97) (Figure 1B). In a multivariate analysis, the PBSC source was significantly associated with higher NRM (OR, 6.55; 95% CI, [1.488-28.820], p = 0.01).
Figure 1: MRD status and clinical outcomes. Cumulative incidence of relapse (1.A), the cumulative incidence of non-relapse mortality (1.B), overall survival (1.C) and event-free survival (1.D).
OS and EFS: At last contact, and after a median follow-up of 25 months (range, 1-205 months) and 47 months (range, 4-205) for surviving patients, 56 patients are alive (65%) in the entire cohort. There was a trend towards better OS in MRD- compared to MRD+ groups (74% vs. 61% respectively, p = 0.07), and no significant difference in EFS between MRD- and MRD+ groups (70% vs. 58% respectively, p = 0.10) (Figure 1C,D). In a multivariate analysis, pre-AHSCT MRD+ status and non-TBI-based conditioning were significantly associated with lower OS (OR, 2.30; 95% CI, [1.027-5.168], p = 0.04 and OR, 3.91; 95% CI, [1.624-9.418], p = 0.002, respectively). The only predicting factor of lower EFS was the non-TBI-based regimen (OR, 2.82; 95% CI, [1.308-6.097], p = 0.008).
Subgroup analysis
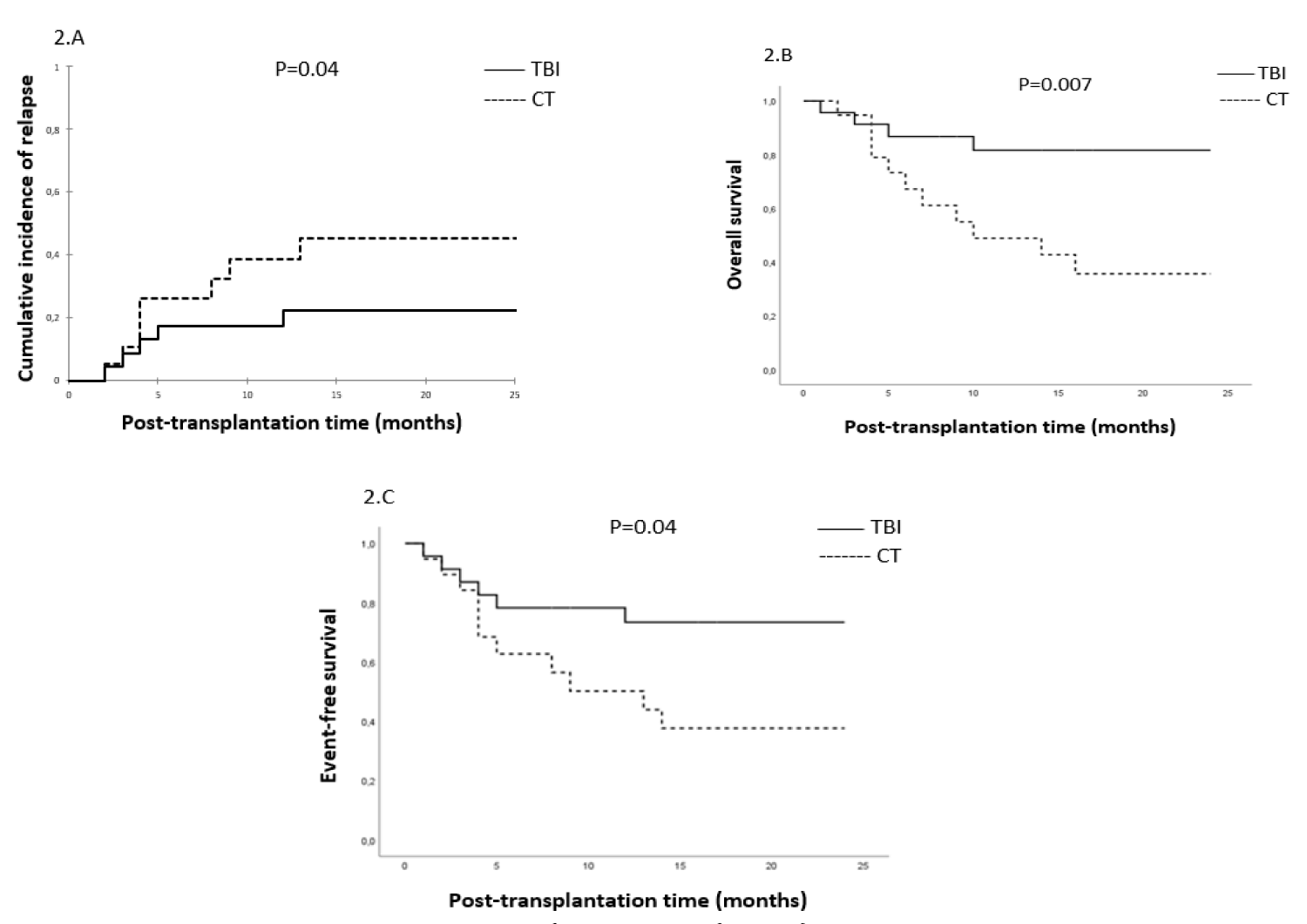
To better describe the impact of the conditioning regimen on OS, EFS, NRM, and CIR, we compared in a subgroup analysis the MRD+ and MRD- patients who received TBI and CT-based regimens. We found that patients with MRD+ status who received TBI-based had significantly lower CIR (22% vs. 60%, p = 0.04) (Figure 2A), better OS (82% vs. 36%, p = 0.007) (Figure 2B) and better EFS (73% vs. 38%, p = 0.04) (Figure 2C). However, in MRD- patients conditioning regimen did not significantly impact OS (86% vs. 60%, p = 0.09) and EFS (81% vs. 58%, p = 0.08). A trend towards lower CIR was observed in the TBI-based regimen (10% vs. 32%, p = 0.06) Tables 2,3.
Figure 2: Subgroup analysis in MRD+ patients: Cumulative incidence of relapse (2.A), overall survival (2.B), and event-free survival (2.C).
| Table 2: Univariate analysis for overall survival, event-free survival, cumulative incidences of relapse, and non-relapse mortality. | ||||||||
| Variables | 2-year OS | 2-year EFS | 2-year CIR | 2-year CI of NRM | ||||
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Gender Male Female |
65% 73% |
0.46 | 61% 70% |
0.40 | 30% 29% |
0.93 | 18% 16% |
0.31 |
| Age < 18years ≥ 18years |
72% 63% |
0.47 | 68% 61% |
0.52 | 35% 24% |
0.21 | 0% 32% |
0.001 |
| ALL subtype B-ALL T-ALL |
64% 73% |
0.50 | 60% 70% |
0.59 | 38% 19% |
0.08 | 17% 16% |
0.37 |
| Karyotype Others/not available High risk |
65% 84% |
0.28 | 61% 85% |
0.18 | 31% 19% |
0.34 | 15% 29% |
0.60 |
| Median time diagnosis-AHSCT (months) < 6months ≥ 6months |
73% 64% |
0.71 | 69% 62% |
0.72 | 16% 36% |
0.08 | 24% 16% |
0.10 |
| Sex mismatch Others Female to male |
69% 66% |
0.72 | 66% 61% |
0.46 | 29% 30% |
0.94 | 19% 14% |
0.72 |
| Remission status at AHSCT CR1 ≥ CR2 |
69% 64% |
0.78 | 68% 53% |
0.36 | 26% 41% |
0.24 | 15% 25% |
0.88 |
| MRD status prior to AHSCT < 0.1% ≥ 0.1% |
74% 61% |
0.07 | 70% 58% |
0.10 | 20% 39% |
0.04 | 15% 15% |
0.97 |
| Conditioning regimen TBI-based CT-based |
84% 48% |
0.002 | 77% 48% |
0.007 | 16% 46% |
0.006 | 11% 22% |
0.31 |
| Stem cells source BM PBSC |
67% 69% |
0.85 | 65% 63% |
0.88 | 32% 22% |
0.27 | 5% 44% |
0.003 |
| Acute GVHD grade II-IV No Yes |
73% 60% |
0.18 | 68% 58% |
0.30 | 35% 21% |
0.25 | 13% 21% |
0.04 |
| Chronic GVHD No Yes |
63% 76% |
0.18 | 59% 73% |
0.14 | 36% 18% |
0.05 | 8% 29% |
0.16 |
| Table 3: Multivariate analysis for overall survival, event-free survival, cumulative incidences of relapse, and non-relapse mortality. | |||||||||
| Variables | 2-year OS | p | 2-year EFS | 2-year CIR | 2-year CI of NRM | ||||
| OR (95% CI) | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |||
| Gender Male Female |
1 0.87(0.340-2.243) |
0.78 | 1 0.82(0.340-1.980) |
0.66 | 1 1.07(0.300-3.790) |
0.92 | - | ||
| Age < 18years ≥ 18years |
1 1.66(0.685-4.007) |
0.26 | 1 1.58(0.694-3.585) |
0.28 | 1 0.84(0.223-3.151) |
0.79 | - | ||
| Remission status at AHSCT CR1 ≥ CR2 |
1 1.79(0.609-5.253) |
0.29 | 1 1.81(0.767-4.296) |
0.17 | 1 4.74(1.197-18.791) |
0.03 | 1 0.57(0.091-3.538) |
0.54 | |
| MRD status prior to AHSCT < 0.1% ≥ 0.1% |
1 2.30(1.027-5.168) |
0.04 | 1 1.92(0.917-4.042) |
0.08 | 1 2.92(0.937-9.079) |
0.06 | 1 1.47(0.331-6.501) |
0.61 | |
| Conditioning regimen TBI-based CT-based |
1 3.91(1.624-9.418) |
0.002 | 1 2.82(1.308-6.097) |
0.008 | 1 6.25(1.947-20.055) |
0.002 | 1 0.80(0.152-4.198) |
0.79 | |
| Stem cells source BM PBSC |
1 0.69(0.238-2.020) |
0.50 | 1 0.69(0.229-2.067) |
0.50 | 1 0.26(0.064-1.035) |
0.05 | 1 6.55(1.488-28.820) |
0.01 | |
| Acute GVHD grade II-IV No Yes |
1 1.65(0.732-3.700) |
0.23 | 1 1.32(0.622-2.796) |
0.47 | 1 0.56(0.170-1.828) |
0.33 | 1 3.46(0.776-15.474) |
0.10 | |
| Chronic GVHD No Yes |
1 0.49(0.206-1.197) |
0.12 | 1 0.52(0.231-1.189) |
0.12 | 1 0.45(0.121-1.676) |
0.23 | 1 1.39(0.306-6.369) |
0.67 | |
| CR: Complete Remission; CR1: First Complete Remission; CR2: Second Complete Remission; MRD: Minimal Residual Disease; CT: Chemotherapy; TBI: Total Body Irradiation; BM: Bone Marrow; PBSC: Peripheral Blood Stem Cells; GVHD: Graft-Versus-Host Disease. | |||||||||
AHSCT remains the standard consolidation treatment of high-risk ALL patients [6]. We retrospectively evaluated the role of MRD status assessed by 4-6 color MFC before AHSCT for high-risk Ph-negative ALL patients. Our data found that pre-AHSCT MRD- status and TBI-based conditioning were significantly associated with better OS. The TBI-based regimen was the only predictive factor of a better EFS. TBI-based conditioning and CR1 were significantly associated with lower CIR. Previous studies showed that high BM MRD level (10-2 – 10-3) before AHSCT was associated with an increased risk of relapse (60% - 100%), whereas deep negative (10-5) and undetectable MRD were associated with 25% - 30% probability of relapse [7-11].
The negative impact of MRD+ prior to AHSCT on clinical outcomes was demonstrated in several studies by either MFC, real-time quantitative-Polymerase Chain Reaction (RQ-PCR), and next-generation sequencing (NGS) techniques with different cutoff values (10-3, 10-4, 10-5 and 10-6) [12-16]. The widely accepted threshold pre-AHSCT MRD to predict better outcomes was < 10-4. Our study found that pre-AHSCT MRD at level ≥ 10-3 was associated with a high risk of relapse with a trend to statistical significance (0.06).
MFC using 4-6 colors was used in our study because it is centralized in the same laboratory, applicable in > 95% of ALL. More sensitive MFC (≥ 8 colors) needs standardization and significant expertise. More sensitive techniques like RQ-PCR and NGS are not available in our country. Despite achieving molecular remission (MRD < 10-4), relapse occurred in 20% - 30% of Ph-negative ALL, probably due to the role of other prognostic factors like genetic abnormalities and clonal evolution [17].
In our study, the TBI-based conditioning was associated with significantly greater OS (84% vs. 48%, p = 0.002), EFS (77% vs. 48%, p = 0.007), and a lower risk of CIR (16% vs. 46%, p = 0.006). The positive impact of TBI on outcomes is particularly evident in patients with MRD+ status for OS (82% vs. 36%, p = 0.007), EFS (73% vs. 38%, p = 0.04), and CIR (22% vs. 60%, p = 0.04). However, in MRD- patients, a trend towards lower CIR was noted in the TBI-based regimen (10% vs. 32%, p = 0.06). TBI was preferred to a CT-based regimen in ALL, especially in high-risk patients [18-20]. According to a recent retrospective study of European Bone Marrow Transplantation (EBMT), worse outcomes were reported after CT-based conditioning for patients with ALL in CR2 [21]. The choice of conditioning regimen could be guided by MRD status prior to AHSCT. According to Cahu X, et al. in a retrospective study, MRD- before AHSCT by NGS technique resulted in a lower risk of relapse irrespective of conditioning, suggesting that TBI may be reserved for patients with positive MRD [22]. A recent international randomized FORUM trial in high-risk pediatric ALL patients confirmed the advantage of TBI in terms of CIR, EFS, OS, and NRM. However, MRD at a level of 10-3 assessed by MFC or PCR did not significantly impact outcomes, probably because most of the patients had an undetectable or very low level of MRD at AHSCT [23].
Our findings should be interpreted with caution because of the observational-retrospective nature of the research, which introduces the possibility of statistical bias. The relatively low number of patients, the large study period with the use of 4 then 6 color MFC with limited sensitivity, the absence of molecular analysis, and the unavailability of MRD status after AHSCT. On the other hand, it has some strong points related to the homogeneity of our population including pediatrics and young adults with high-risk Ph-negative ALL receiving myeloablative conditioning regimen, matching sibling donors, and the centralization of the MRD analysis. However, despite these limitations, our study showed a higher CIR among patients with MRD levels prior to AHSCT ≥ 10-3 by MFC and suggests the indication of immunotherapy prior to and/or earlier after AHSCT.
We didn’t find a significant impact of MRD at the threshold of 10-4 (data not shown) this may be explained by the low number of bone marrow cells prior to AHSCT, as well as the low sensitivity of 4 colors panel. Association of LAIP-based and different from normal (DFN) approach and a better combination of markers included in Euroflow ≥ 8 colors standardized panels are actually investigated to improve and perform the applicability and sensitivity of MRD assessment in our country.
Current standard recommendations advise giving Blinatumomab a CD3/CD19-directed bispecific T-cell engager molecule before AHSCT to high-risk patients with MRD [24]. With a long-term follow-up, the BLAST trial confirms the potentially curative treatment by Blinatumomab in adults with B-ALL with MRD ≥ 10-3 level, consolidated with or without AHSCT [25]. Other immunologic approaches including rapidly withdrawn immune suppression and donor Lymphocyte Infusion after AHSCT, have limited efficacy and a higher risk of GVHD [26,27]. The role of CAR-T cells as a bridge to AHSCT remains controversial. Further new treatment approaches are urgently needed for T-ALL.
MRD level ≥ 10-3 prior AHSCT was associated with lower OS and tends towards higher CIR and guides TBI-based conditioning to improve outcomes. The use of immunotherapy, highly effective in MRD settings including Blinatumomab may provide better survival and lower relapse.
The authors thank all medical staff who supported this study and participated in the management of the patients.
- Giebel S, Labopin M, Socié G, Beelen D, Browne P, Volin L, Kyrcz-Krzemien S, Yakoub-Agha I, Aljurf M, Wu D, Michallet M, Arnold R, Mohty M, Nagler A. Improving results of allogeneic hematopoietic cell transplantation for adults with acute lymphoblastic leukemia in first complete remission: an analysis from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica. 2017 Jan;102(1):139-149. doi: 10.3324/haematol.2016.145631. Epub 2016 Sep 29. PMID: 27686376; PMCID: PMC5210244..
- Varadarajan I, Pierce E, Scheuing L, Morris A, El Chaer F, Keng M. Post-Hematopoietic Cell Transplantation Relapsed Acute Lymphoblastic Leukemia: Current Challenges and Future Directions. Onco Targets Ther. 2023 Jan 14;16:1-16. doi: 10.2147/OTT.S274551. PMID: 36685611; PMCID: PMC9849790.
- Short NJ, Jabbour E, Albitar M, de Lima M, Gore L, Jorgensen J, Logan AC, Park J, Ravandi F, Shah B, Radich J, Kantarjian H. Recommendations for the assessment and management of measurable residual disease in adults with acute lymphoblastic leukemia: A consensus of North American experts. Am J Hematol. 2019 Feb;94(2):257-265. doi: 10.1002/ajh.25338. Epub 2018 Nov 26. PMID: 30394566; PMCID: PMC6572728.
- Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, Lerner KG, Thomas ED. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974 Oct;18(4):295-304. doi: 10.1097/00007890-197410000-00001. PMID: 4153799.
- Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, Hackman R, Tsoi MS, Storb R, Thomas ED. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980 Aug;69(2):204-17. doi: 10.1016/0002-9343(80)90380-0. PMID: 6996481.
- Beelen DW, Arnold R, Stelljes M, Alakel N, Brecht A, Bug G, Bunjes D, Faul C, Finke J, Franke GN, Holler E, Kobbe G, Kröger N, Rösler W, Scheid C, Schönland S, Stadler M, Tischer J, Wagner-Drouet E, Wendelin K, Brüggemann M, Reiser L, Hoelzer D, Gökbuget N. Long-Term Results of Allogeneic Stem Cell Transplantation in Adult Ph- Negative High-Risk Acute Lymphoblastic Leukemia. Transplant Cell Ther. 2022 Dec;28(12):834-842. doi: 10.1016/j.jtct.2022.08.024. Epub 2022 Aug 27. PMID: 36031078.
- Knechtli CJ, Goulden NJ, Hancock JP, Grandage VL, Harris EL, Garland RJ, Jones CG, Rowbottom AW, Hunt LP, Green AF, Clarke E, Lankester AW, Cornish JM, Pamphilon DH, Steward CG, Oakhill A. Minimal residual disease status before allogeneic bone marrow transplantation is an important determinant of successful outcome for children and adolescents with acute lymphoblastic leukemia. Blood. 1998 Dec 1;92(11):4072-9. PMID: 9834212.
- Pulsipher MA, Langholz B, Wall DA, Schultz KR, Bunin N, Carroll WL, Raetz E, Gardner S, Gastier-Foster JM, Howrie D, Goyal RK, Douglas JG, Borowitz M, Barnes Y, Teachey DT, Taylor C, Grupp SA. The addition of sirolimus to tacrolimus/methotrexate GVHD prophylaxis in children with ALL: a phase 3 Children's Oncology Group/Pediatric Blood and Marrow Transplant Consortium trial. Blood. 2014 Mar 27;123(13):2017-25. doi: 10.1182/blood-2013-10-534297. Epub 2014 Feb 4. PMID: 24497539; PMCID: PMC3968388.
- Lovisa F, Zecca M, Rossi B, Campeggio M, Magrin E, Giarin E, Buldini B, Songia S, Cazzaniga G, Mina T, Acquafredda G, Quarello P, Locatelli F, Fagioli F, Basso G. Pre- and post-transplant minimal residual disease predicts relapse occurrence in children with acute lymphoblastic leukaemia. Br J Haematol. 2018 Mar;180(5):680-693. doi: 10.1111/bjh.15086. Epub 2018 Jan 23. PMID: 29359790.
- Bader P, Salzmann-Manrique E, Balduzzi A, Dalle JH, Woolfrey AE, Bar M, Verneris MR, Borowitz MJ, Shah NN, Gossai N, Shaw PJ, Chen AR, Schultz KR, Kreyenberg H, Di Maio L, Cazzaniga G, Eckert C, van der Velden VHJ, Sutton R, Lankester A, Peters C, Klingebiel TE, Willasch AM, Grupp SA, Pulsipher MA. More precisely defining risk peri-HCT in pediatric ALL: pre- vs post-MRD measures, serial positivity, and risk modeling. Blood Adv. 2019 Nov 12;3(21):3393-3405. doi: 10.1182/bloodadvances.2019000449. PMID: 31714961; PMCID: PMC6855112.
- Holowiecki J, Krawczyk-Kulis M, Giebel S, Jagoda K, Stella-Holowiecka B, Piatkowska-Jakubas B, Paluszewska M, Seferynska I, Lewandowski K, Kielbinski M, Czyz A, Balana-Nowak A, Król M, Skotnicki AB, Jedrzejczak WW, Warzocha K, Lange A, Hellmann A. Status of minimal residual disease after induction predicts outcome in both standard and high-risk Ph-negative adult acute lymphoblastic leukaemia. The Polish Adult Leukemia Group ALL 4-2002 MRD Study. Br J Haematol. 2008 Jun;142(2):227-37. doi: 10.1111/j.1365-2141.2008.07185.x. Epub 2008 May 19. PMID: 18492099.
- Ravandi F, Jorgensen JL, O'Brien SM, Jabbour E, Thomas DA, Borthakur G, Garris R, Huang X, Garcia-Manero G, Burger JA, Ferrajoli A, Wierda W, Kadia T, Jain N, Wang SA, Konoplev S, Kebriaei P, Champlin RE, McCue D, Estrov Z, Cortes JE, Kantarjian HM. Minimal residual disease assessed by multi-parameter flow cytometry is highly prognostic in adult patients with acute lymphoblastic leukaemia. Br J Haematol. 2016 Feb;172(3):392-400. doi:10.1111/bjh.13834. Epub 2015 Oct 22. PMID: 26492205; PMCID: PMC4826052.
- Dhédin N, Huynh A, Maury S, Tabrizi R, Beldjord K, Asnafi V, Thomas X, Chevallier P, Nguyen S, Coiteux V, Bourhis JH, Hichri Y, Escoffre-Barbe M, Reman O, Graux C, Chalandon Y, Blaise D, Schanz U, Lhéritier V, Cahn JY, Dombret H, Ifrah N; GRAALL group. Role of allogeneic stem cell transplantation in adult patients with Ph-negative acute lymphoblastic leukemia. Blood. 2015 Apr 16;125(16):2486-96; quiz 2586. doi: 10.1182/blood-2014-09-599894. Epub 2015 Jan 13. PMID: 25587040.
- Gökbuget N, Kneba M, Raff T, Trautmann H, Bartram CR, Arnold R, Fietkau R, Freund M, Ganser A, Ludwig WD, Maschmeyer G, Rieder H, Schwartz S, Serve H, Thiel E, Brüggemann M, Hoelzer D; German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia. Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood. 2012 Aug 30;120(9):1868-76. doi: 10.1182/blood-2011-09-377713. Epub 2012 Mar 22. PMID: 22442346.
- Pulsipher MA, Carlson C, Langholz B, Wall DA, Schultz KR, Bunin N, Kirsch I, Gastier-Foster JM, Borowitz M, Desmarais C, Williamson D, Kalos M, Grupp SA. IgH-V(D)J NGS-MRD measurement pre- and early post-allotransplant defines very low- and very high-risk ALL patients. Blood. 2015 May 28;125(22):3501-8. doi: 10.1182/blood-2014-12-615757. Epub 2015 Apr 10. PMID: 25862561; PMCID: PMC4447864.
- Ribera JM, Oriol A, Morgades M, Montesinos P, Sarrà J, González-Campos J, Brunet S, Tormo M, Fernández-Abellán P, Guàrdia R, Bernal MT, Esteve J, Barba P, Moreno MJ, Bermúdez A, Cladera A, Escoda L, García-Boyero R, Del Potro E, Bergua J, Amigo ML, Grande C, Rabuñal MJ, Hernández-Rivas JM, Feliu E. Treatment of high-risk Philadelphia chromosome-negative acute lymphoblastic leukemia in adolescents and adults according to early cytologic response and minimal residual disease after consolidation assessed by flow cytometry: final results of the PETHEMA ALL-AR-03 trial. J Clin Oncol. 2014 May 20;32(15):1595-604. doi: 10.1200/JCO.2013.52.2425. Epub 2014 Apr 21. PMID: 24752047.
- Enshaei A, O'Connor D, Bartram J, Hancock J, Harrison CJ, Hough R, Samarasinghe S, den Boer ML, Boer JM, de Groot-Kruseman HA, Marquart HV, Noren-Nystrom U, Schmiegelow K, Schwab C, Horstmann MA, Escherich G, Heyman M, Pieters R, Vora A, Moppett J, Moorman AV. A validated novel continuous prognostic index to deliver stratified medicine in pediatric acute lymphoblastic leukemia. Blood. 2020 Apr 23;135(17):1438-1446. doi: 10.1182/blood.2019003191. Erratum in: Blood. 2020 Sep 17;136(12):1468. PMID: 32315382.
- Sanchez-Garcia J, Serrano J, Serrano-Lopez J, Gomez-Garcia P, Martinez F, Garcia-Castellano JM, Rojas R, Martin C, Rodriguez-Villa A, Molina-Hurtado JR, Alvarez MA, Casaño J, Torres-Gomez A. Quantification of minimal residual disease levels by flow cytometry at time of transplant predicts outcome after myeloablative allogeneic transplantation in ALL. Bone Marrow Transplant. 2013 Mar;48(3):396-402. doi: 10.1038/bmt.2012.147. Epub 2012 Jul 30. PMID: 22858507.
- Eroglu C, Pala C, Kaynar L, Yaray K, Aksozen MT, Bankir M, Zararsız G, Orhan O, Gündog M, Yıldız OG, Eser B, Cetin M, Unal A. Comparison of total body irradiation plus cyclophosphamide with busulfan plus cyclophosphamide as conditioning regimens in patients with acute lymphoblastic leukemia undergoing allogeneic hematopoietic stem cell transplant. Leuk Lymphoma. 2013 Nov;54(11):2474-9. doi: 10.3109/10428194.2013.779691. Epub 2013 Mar 27. PMID: 23442062.
- Cahu X, Labopin M, Giebel S, Aljurf M, Kyrcz-Krzemien S, Socié G, Eder M, Bonifazi F, Bunjes D, Vigouroux S, Michallet M, Stelljes M, Zuckerman T, Finke J, Passweg J, Yakoub-Agha I, Niederwieser D, Sucak G, Sengeløv H, Polge E, Nagler A, Esteve J, Mohty M; Acute Leukemia Working Party of EBMT. Impact of conditioning with TBI in adult patients with T-cell ALL who receive a myeloablative allogeneic stem cell transplantation: a report from the acute leukemia working party of EBMT. Bone Marrow Transplant. 2016 Mar;51(3):351-7. doi: 10.1038/bmt.2015.278. Epub 2015 Nov 30. PMID: 26618548.
- Pavlů J, Labopin M, Niittyvuopio R, Socié G, Yakoub-Agha I, Wu D, Remenyi P, Passweg J, Beelen DW, Aljurf M, Kröger N, Labussière-Wallet H, Perić Z, Giebel S, Nagler A, Mohty M. Measurable residual disease at myeloablative allogeneic transplantation in adults with acute lymphoblastic leukemia: a retrospective registry study on 2780 patients from the acute leukemia working party of the EBMT. J Hematol Oncol. 2019 Oct 23;12(1):108. doi: 10.1186/s13045-019-0790-x. PMID: 31647022; PMCID: PMC6813121.
- Friend BD, Bailey-Olson M, Melton A, Shimano KA, Kharbanda S, Higham C, Winestone LE, Huang J, Stieglitz E, Dvorak CC. The impact of total body irradiation-based regimens on outcomes in children and young adults with acute lymphoblastic leukemia undergoing allogeneic hematopoietic stem cell transplantation. Pediatr Blood Cancer. 2020 Feb;67(2):e28079. doi: 10.1002/pbc.28079. Epub 2019 Nov 14. PMID: 31724815.
- Peters C, Dalle JH, Locatelli F, Poetschger U, Sedlacek P, Buechner J, Shaw PJ, Staciuk R, Ifversen M, Pichler H, Vettenranta K, Svec P, Aleinikova O, Stein J, Güngör T, Toporski J, Truong TH, Diaz-de-Heredia C, Bierings M, Ariffin H, Essa M, Burkhardt B, Schultz K, Meisel R, Lankester A, Ansari M, Schrappe M; IBFM Study Group;; von Stackelberg A; IntReALL Study Group; Balduzzi A; I-BFM SCT Study Group; Corbacioglu S; EBMT Paediatric Diseases Working Party; Bader P. Total Body Irradiation or Chemotherapy Conditioning in Childhood ALL: A Multinational, Randomized, Noninferiority Phase III Study. J Clin Oncol. 2021 Feb 1;39(4):295-307. doi: 10.1200/JCO.20.02529. Epub 2020 Dec 17. PMID: 33332189; PMCID: PMC8078415.
- Gökbuget N, Dombret H, Bonifacio M, Reichle A, Graux C, Faul C, Diedrich H, Topp MS, Brüggemann M, Horst HA, Havelange V, Stieglmaier J, Wessels H, Haddad V, Benjamin JE, Zugmaier G, Nagorsen D, Bargou RC. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018 Apr 5;131(14):1522-1531. doi: 10.1182/blood-2017-08-798322. Epub 2018 Jan 22. Erratum in: Blood. 2019 Jun 13;133(24):2625. PMID: 29358182; PMCID: PMC6027091.
- Gökbuget N, Zugmaier G, Dombret H, Stein A, Bonifacio M, Graux C, Faul C, Brüggemann M, Taylor K, Mergen N, Reichle A, Horst HA, Havelange V, Topp MS, Bargou RC. Curative outcomes following blinatumomab in adults with minimal residual disease B-cell precursor acute lymphoblastic leukemia. Leuk Lymphoma. 2020 Nov;61(11):2665-2673. doi: 10.1080/10428194.2020.1780583. Epub 2020 Jul 3. PMID: 32619115.
- Dominietto A, Piaggio G, MD, Pozzi S, MD, Bertolotti F, Colombo N, Grasso R, Garuti A, Cirmena G, Miglino M, Raiola AM, Van Lint MT, Frassoni F, Podesta’ M, Bacigalupo A. Treatment of Minimal Residual Disease (MRD) with Donor Lymphocyte Infusions (DLI) in Acute Leukemia Patients Undergoing an Allogeneic Hemopoietic Stem Cell Transplants (HSCT). Blood. 2005 Nov;106(11):2012. doi:10.1182/blood.V106.11.2012.2012.
- Yan CH, Liu QF, Wu DP, Zhang X, Xu LP, Zhang XH, Wang Y, Huang H, Bai H, Huang F, Ma X, Huang XJ. Prophylactic Donor Lymphocyte Infusion (DLI) Followed by Minimal Residual Disease and Graft-versus-Host Disease-Guided Multiple DLIs Could Improve Outcomes after Allogeneic Hematopoietic Stem Cell Transplantation in Patients with Refractory/Relapsed Acute Leukemia. Biol Blood Marrow Transplant. 2017 Aug;23(8):1311-1319. doi: 10.1016/j.bbmt.2017.04.028. Epub 2017 May 5. Erratum in: Biol Blood Marrow Transplant. 2020 Jan;26(1):214. PMID: 28483716.