Research Article
Preliminary Report on the Effect of Mesenchymal Stem Cell Therapy in Patients with Chronic Lung Allograft Dysfunction

Cesar A Keller1*, Thomas A Gonwa1, Athena L Russell2, David O Hodge3, David B Erasmus1 and Abba C Zubair2
1Division of Transplant Medicine, Mayo Clinic, 4500 San Pablo Rd, Jacksonville, Florida, USA2Department of Laboratory Medicine & Pathology, Mayo Clinic, Jacksonville, Florida, USA
3Division of Biomedical Statistics and Informatics, Mayo Clinic, Jacksonville, Florida, USA
*Address for Correspondence: Cesar A Keller, Division of Transplant Medicine, Mayo Clinic, 4500 San Pablo Rd, Jacksonville, FL 32224, Florida, USA, Tel: 904-956-3271; Fax: 904-956-3262; Email: keller.cesar@mayo.edu
Dates: Submitted: 14 August 2018; Approved: 27 August 2018; Published: 28 August 2018
How to cite this article: Keller CA, Gonwa TA, Russell AL, Hodge DO, Erasmus DB, et al. Preliminary Report on the Effect of Mesenchymal Stem Cell Therapy in Patients with Chronic Lung Allograft Dysfunction. J Stem Cell Ther Transplant. 2018; 2: 035-047. DOI: 10.29328/journal.jsctt.1001012
Copyright License: © 2018 Keller CA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Bronchiolitis obliterans syndrome; Lung transplantation; Mesenchymal stem cells; Obstructive CLAD
Abbreviations: BOS: Bronchiolitis Obliterans Syndrome; CLAD: Chronic Lung Allograft Dysfunction; FEV1: Forced Expiratory Volume in 1 second; FVC: Forced Vital Capacity; IL: Inter Leukin; MSC: Mesenchymal Stem Cell; NK: Natural Killer; Th: T Helper; Tregs: Regulatory T Cells
Abstract
Background: Mesenchymal stem cell (MSC) effects can shift immune responses toward anti-inflammatory and tolerogenic phenotypes, potentially helping patients with bronchiolitis obliterans syndrome (BOS).
Methods: We evaluated the effect of infusing allogeneic MSC intravenously in 9 patients with moderate BOS refractory to standard therapy who were not candidates for retransplant, dividing them into 3 dosing groups: Group 1, 1×106 MSC/kg (n=3); Group 2, 2×106 MSC/kg (n=3); and Group 3, 4×106 MSC/kg (n=3). We recorded pulmonary function tests, laboratory variables, and serum biomarkers pre- and post-MSC infusion.
Results: These patients had significant decline in forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1) over 1 year pre-MSC infusion (mean ± SD) FVC, 3.11±0.98 L, and FEV1 1.99+0.64 L versus FVC 2.58±1.03 and FEV1 1.61±0.52 just before infusion (P<0.05); representing a mean loss of 530 mL in FVC and 374 mL in FEV1 over 12 months. One year post-MSC infusion, mean FVC and FEV1 increased to 2.66±1.01 L and 1.63±0.55 L, respectively (changes no longer significant compared to before MSC infusion). Patients in Group 1 showed elevation of tolerance-inducing T regulatory cells and increased levels of epidermal growth factor. Tolerance-inducing Th-2 cytokines increased in Groups 1 and 2. These changes were not significantly different in these small sub-groups.
Conclusion: MSC infusion appears to slow down or reverse the progressive decline in lung function in some patients with moderate BOS, possibly by inducing anti-inflammatory effects and promoting cell proliferation and angiogenesis.
Introduction
Lung transplantation is the best option of care for a variety of end-stage lung conditions no longer responding to standard options of medical or surgical care [1]. The most common causes of early morbidity and mortality after transplant include primary graft dysfunction [2], acute infectious complications [3], acute cellular rejection [4], or acute antibody-mediated rejection [5]. Long-term survival of lung transplant recipients is compromised by chronic lung allograft dysfunction (CLAD) [6], either obstructive (or bronchiolitis obliterans syndrome [BOS]) [7] or restrictive (restrictive allograft syndrome) [8].
Patients with BOS develop progressive decline in lung airflow measured by the forced expiratory volume in 1 second (FEV1), following the peak post-transplant FEV1 achieved, not concomitantly related to acute rejection, airway stenosis, or acute infection [7]. This effect is likely predisposed by repeated previous episodes of acute rejection; ischemic and vascular injury; bacterial, fungal, or viral infections; active gastroesophageal reflux; environmental exposures; and others [9]. Although some patients stabilize or improve after enhanced immunosuppression [10,11], addition of azithromycin [12], medical or surgical treatment of gastro esophageal reflux disease [13], or extracorporeal photopheresis, a large portion of these patients will continue to have severe advanced disability or death, unless they receive a repeat transplant [14]. Unfortunately, retransplant has a lower rate of survival than initial transplant [15], and not every patient will qualify to receive a second transplant. Alternatives of care for patients with refractory BOS are limited; therefore, continued exploration of further options of care are justified. Mesenchymal stem cells (MSCs) are known to modulate the cellular immune system suppressing effector T cells [16]. They are known to shift immune responses toward anti-inflammatory and tolerogenic phenotypes, shifting from T helper (Th) 1 to Th2 immune response [17]. MSCs also reduce antibody production by B cells [18]. In addition, MSCs has been shown to be naturally and preferentially trapped in the lung [19], providing the cells easy access into lung tissue, particularly if there is a process of active inflammation present [20]. Clinical trials have evaluated autologous and allogeneic MSCs in the treatment of inflammatory conditions, such as graft-versus-host disease [21], and preliminary results have shown potential, beneficial functional effect using MSCs to treat lung transplant recipients with obstructive CLAD in a small group of patients [22,23].
Our group and others have also demonstrated that intravenous administration of bone marrow-derived allogeneic MSC is safe and well tolerated by lung transplant recipients diagnosed with moderate or severe BOS [22,24].
In our phase 1 study, we evaluated the functional outcome in 9 patients diagnosed with moderate BOS, refractory to standard medical therapy during the first year after receiving MSC therapy. Patients received a single infusion of bone marrow-derived allogeneic MSCs using a dose escalation model. In addition, we measured biomarkers in blood samples of these patients to evaluate changes to immune cell, cytokine, and growth factor levels before and after intravenous infusion of MSCs.
Materials and Methods
This study was approved by the Mayo Clinic Institutional Review Board (Protocol#14-000025; Investigational New Drug#15897). The trial was registered at ClinicalTrials.gov [25].
Patient population
Study patients were recruited from the lung transplant program at Mayo Clinic in Jacksonville, Florida. Patients diagnosed with moderate BOS, refractory to standard medical therapy, who did not qualify for retransplant were offered study participation and enrolled after providing informed consent. Inclusion and exclusion criteria, process of enrollment, manufacturing, transportation, and preparations for infusion of allogeneic bone marrow-derived MSCs have all been reported in a previous publication [24]. All patients received MSC manufactured by Waisman Biomanufacturing at the University of Wisconsin-Madison. Bone marrow was harvested from a young healthy male (18-45 years), blood type O who completed a standardized Donor Health History Questionnaire and had communicable disease testing in accordance with FDA standard 21 CFR part 1271-subpart C Donor Eligibility Final Rule. Allogeneic bone marrow derived MSC were manufactured using standard manual tissue culture flasks, cryopreserved bags were stored and shipped to our facility where they remained frozen until infusion day where the frozen MSCs underwent standard protocol for thawing, 5x dilution and infusion [24]. Patients were enrolled from September 1, 2014, through October 31, 2015, and each patient was followed for up to a year after infusion of MSCs.
Patients were assigned to a dose-escalation model to receive a single infusion of bone marrow-derived MSCs obtained from a single healthy donor as previously described [24]. The first 3 patients were assigned to receive 1×106 MSC/kg (Group 1), the next 3 received 2×106 MSC/kg (Group 2), and the final 3 received 4×106 MSC/kg (Group 3).
Biomarkers monitoring
The day of infusion was considered Day 0. Blood samples were collected at baseline (Days: 1 to 7 or 0 prior to infusion) and on Days 1 and 7 after infusion to enumerate immune cells (B cells, natural killer [NK] cells, regulatory T cells [Tregs]) using flow cytometry and cytokines and growth factors using multiplex assay. These patients were not having any signs of active infections at the time of the Cytokine assay blood draw.
Flow cytometry
Antibody cocktails were used to detect positive and negative MSC surface markers by following International Society for Cellular Therapy guidelines (positive markers: CD90, CD73, and CD105; negative markers: CD45, CD34, CD14 or CD11b, CD79α or CD19, and HLA-DR) (BD Biosciences, Franklin Lane, NJ, Cat. No.562245). Cells coexpressing CD90, CD73, and CD105 while lacking expression of negative markers were considered MSC and were quantified. In addition, mononuclear cells from the blood samples were evaluated for B cells (CD45+ CD19+) (BD Bioscience, Franklin Lane, NJ, Cat. No. 555483 & 555415), NK cells (CD45+ CD56+) (BD Bioscience, Franklin Lane, NJ, Cat.No.555483 &555518), and Tregs (CD4+ CD25+) (BD Bioscience, Franklin Lane, NJ, Cat. No.555346 & 555434).
Cytokine multiplex assay
Plasma was analyzed for the presence and concentration of 42 different cytokines and growth factors using the Human Cytokine Array/Chemokine Array 42-plex Discovery Assay and performed by Eve Technologies. We used the multiplex cytokine assay to assess proinflammatory (Th1) and tolerogenic (Th2) cytokine levels and pro-angiogenic factors such as VEGF (vascular endothelial growth factor).
Clinical monitoring
Patient hemoglobin, white blood cell and platelet counts, blood urea nitrogen, serum creatinine, and glucose levels were measured for all patients prior to infusion (Days 1-7) and on Days 30 and 365 after infusion. Arterial blood gases were obtained prior to infusion and repeated on Days 30 and 365 after infusion. Spirometric values (forced vital capacity [FVC] and FEV1) were obtained on Days -365 and -180 prior to infusion, on Day 0 (just prior MSC to infusion), and on Days 180 and 365 after infusion. Spirometric values obtained on Days -365, -180, 180 and 365 were compared to the values obtained on Day 0 just prior infusion of MSCs. All patients had routine post-lung transplant monitoring with scheduled clinic visits every 3 months and when clinically indicated.
Statistical analysis
All variables are summarized as mean±SD or median (range). Individual variables were compared over time using paired t test. All results were completed using SAS, version 9.4 (SAS Institute Inc.)
Results
Patient population
Demographic characteristics of the patient population are described on Table 1. All enrolled patients had failed to adequately respond to standard medical therapy and were not candidates for repeat transplant. Nine patients (7 men and 2 women) who were recipients of either double-lung (n=5) or single-lung transplant (n=4) were included. They were of advanced age (69±5 years), likely related to the study design excluding younger patients who could qualify for retransplant. Indications for double-lung transplant were idiopathic pulmonary fibrosis (n=2), chronic obstructive pulmonary disease (n=1), primary ciliary dyskinesia (n=1), and a retransplant due to CLAD (n=1). Indications for single-lung transplant were chronic obstructive pulmonary disease (n=3) and idiopathic pulmonary fibrosis (n=1). All had moderate BOS diagnosed a mean of 4.8±2.2 years after transplant. Their mean FEV1 prior to MSC infusion was 1.62±0.51 L which was 56.8%±3.2% of the peak FEV1 achieved posttransplant. This represented a 43.2±3.4% drop in post-transplant FEV1 consistent with moderate BOS. These patients received their MSC infusion at 6.6±3.1 years after lung transplant.
| Table 1: Demographic Characteristics of the 3 Study Groups (N=9). | |||||||||
| Time (y) | FEV1 (L) | FEV1 | FEV1 | Time (y) | |||||
| Tx. To | Day of | from Peak | percent | Tx. To | |||||
| Age | Sex | Diagnosis | Type Tx | BOS 2 | Infusion | Post-Tx. | decline | Infusion | |
| Group 1 | 75 | F | COPD | Single | 5.5 | 1.12 | 53% | 47% | 8.7 |
| 75 | M | COPD | Single | 4.6 | 1.25 | 62% | 38% | 5.0 | |
| 70 | M | IPF | Single | 9.0 | 1.58 | 59% | 41% | 9.5 | |
| Group 2 | 63 | F | BOS | Double* | 3.4 | 1.47 | 59% | 41% | 3.6 |
| 74 | M | COPD | Single | 5.9 | 1.16 | 56% | 44% | 11.7 | |
| 71 | M | PCD | Double | 6.0 | 2.68 | 53% | 47% | 7.2 | |
| Group 3 | 73 | M | COPD | Double | 2.4 | 2.16 | 60% | 40% | 5.9 |
| 59 | M | IPF | Double | 1.4 | 1.51 | 53% | 47% | 1.8 | |
| 61 | M | IPF | Double | 5.0 | 1.61 | 56% | 44% | 5.4 | |
| Mean+SD | 69+6.3 | M=7, F=2 | S=4-D=5 | 4.8+2.2 | 1.62+0.5 | 56.8+3.4 | 43.2+3.4 | 6.6+3.1 | |
| Abbreviations: BOS- Bronchiolitis Obliterans Syndrome; COPD- Chronic Obstructive Pulmonary Disease; F- Female; FEV1- Forced Expiratory Volume in 1 second; IPF- Idiopathic Pulmonary Fibrosis; M- Male; Max- Maximum; Min- Minimum; MSC- Mesenchymal Stem Cells; PCD- Primary Ciliary Dyskinesia; Tx- Transplant. *Redo transplant | |||||||||
Fate of Infused MSC
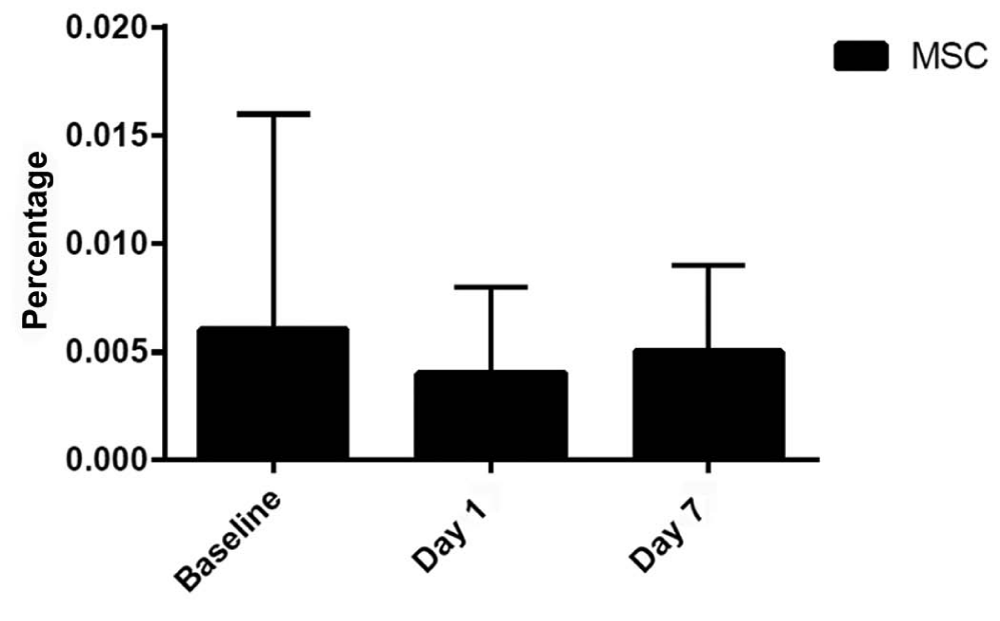
To determine the fate of the infused MSCs, we performed flow cytometric analysis of mononuclear cells from collected blood samples. Figure 1 shows the number of MSCs (mean±SD) at baseline (before MSC infusion) and Days 1 and 7 after infusion for all 9 patients. There was no statistical difference in the number of circulating MSCs, suggesting that all intravenously infused MSCs were trapped in the lungs because we observed no increase in circulating MSCs by 24 hours after infusion.
Figure 1: Percentage of Circulating MSC at Baseline and Days 1 and 7 After Intravenous Infusion for All Groups as mean values (bars) plus standard deviation shown as the line extending up from the main bars. MSC- Mesenchymal Stem Cells.
Effect of MSC therapy on immune cells
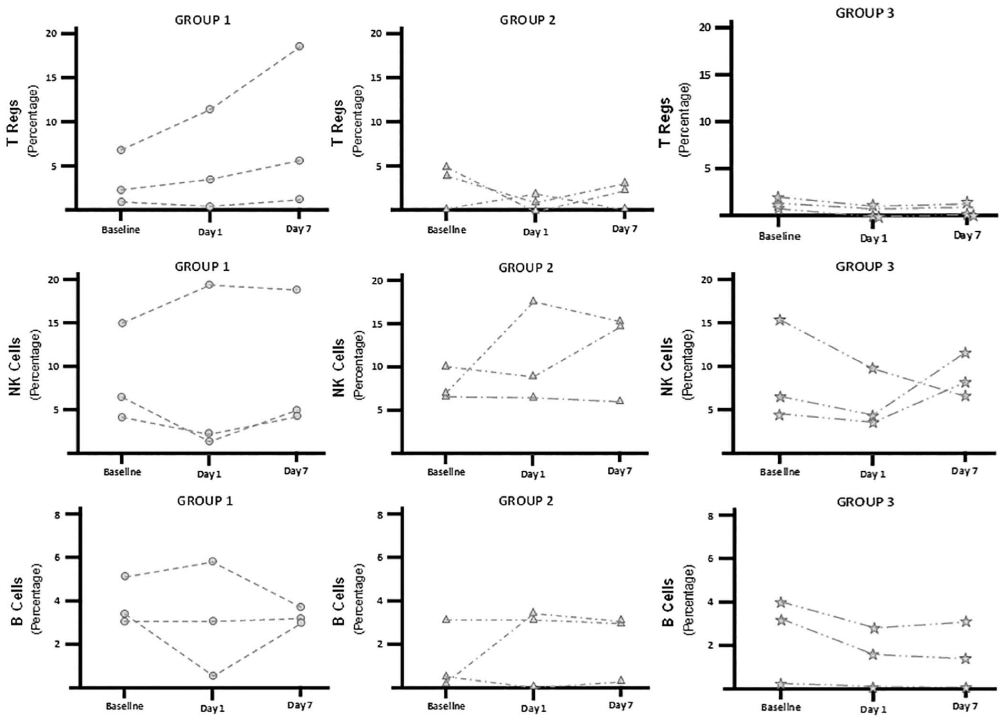
We performed flow cytometric analysis and evaluated the immune effector cells (B, NK, and Treg) in the blood samples, comparing them to baseline levels Figure 2.There was considerable variability at baseline. In Group 1 (low dose), the mean percentage Treg levels increased 2.3 fold relative to baseline by Day 7, this pattern appeared different than in the other groups but without reaching statistical difference. Analysis on B cells and NK cells did not disclose a discernible pattern.
Figure 2: Circulating Immune Cells Stratified By MSC Dose Groups at Baseline and Days 1 and 7 After Infusion. Bars represent mean values and the lines extending up from the main bars indicate standard deviation. Group 1 received 1×106 MSC/kg; Group 2, received 2×106 MSC/kg; and Group 3, received 4×106 MSC/kg. MSC- Mesenchymal Stem Cells; NK- Natural Killer Cells; T Regs- Regulatory T Cells.
Effect of MSC therapy on cytokine profile
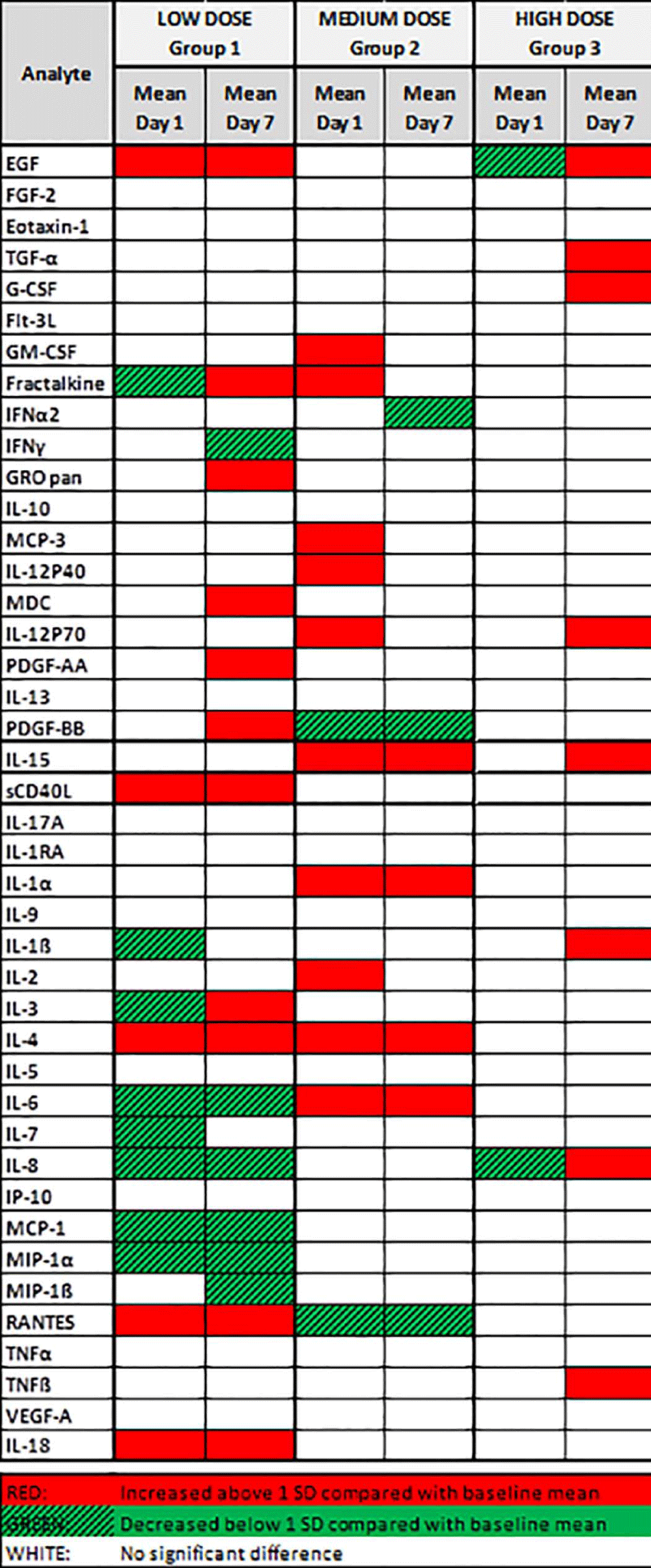
Plasma was analyzed for the presence and concentration of 42 secreted cytokines and chemokines. The results of this analysis are summarized in Figure 3. Changes in cytokine levels greater or less than 1 SD from mean baseline levels were considered significant. Overall, compared with baseline, more cytokines and growth factors appeared to be affected on patients receiving low doses of MSC (Group 1). Promoters of cell proliferation, like epidermal growth factor, which have antiapoptotic and proangiogenic properties, were significantly increased in Group 1. Tolerance-inducing Th2 cytokines, such as interleukin (IL)-4, significantly increased in Groups 1 and 2. Proinflammatory Th1 cytokines (IL-1α, IL-6, and IL-8) and proinflammatory chemokines (monocyte chemoattractant protein (MCP-1), macrophage inflammatory protein-1α (MIP-1α), and MIP-1β) were significantly decreased in Group 1. Overall, the most beneficial biologic effect of MSC therapy appeared to occur in patients receiving the lowest MSC dose compared to the other 2 groups.
Figure 3: Cytokines and Growth Factors Profile By MSC Dose Groups. Group 1 received 1×106 MSC/kg; Group 2, received 2×106 MSC/kg; and Group 3, received 4×106 MSC/kg. Plasma was analyzed for the presence and concentration of 42 secreted human cytokines and chemokines. Changes in cytokine levels greater or less than 1 SD from the mean baseline levels were considered significant. Overall, the number of cytokines and growth factors affected appeared to decrease with increasing MSC dose.
EGF- Epidermal Growth Factor; FGF-2- Fibroblast Growth Factor 2; Flt-3L- Fms-Like Tyrosine Kinase 3 Ligand; G-CSF- Granulocyte Colony Stimulating Factor; GM-CSF- Granulocyte Monocyte Colony Stimulating Factor; GRO pan- Growth-Related Onconge; IL- Interleukin; INFα2- Interferon α 2; INFγ- Interferon γ; IP 10- Interferon γ-Induced Protein; MCP- Monocyte Chemoattractant Protein; MIP- Macrophage Inflammatory Protein; PDGF- Platelet-Derived Growth Factor; AA- Two a sub-units; BB- Two b sub-units; RA- Receptor Antagonist; RANTES- Regulated on activation normal T cell expressed and secreted; sCD40L- Soluble Cluster of differentiation 40 ligand; TGFα- Tumor growth factor α; TNFα- Tumor Necrosis Factor α; TNFβ- Tumor Necrosis Factor β; VEGF-A- Vascular Endothelial growth factor A.
Effect of MSC therapy on blood and renal function
Table 2 displays results from blood work obtained on Day 0 prior to MSC infusion, and then on Days 7, 30 and 365 after MSC infusion. Baseline values revealed a population with normal mean hemoglobin (13.2±1.2 g/dL), white blood cell (6.5±2.0×109 cells/L), and platelet counts (173±39×109 cells/L). Our cohort of 9 patients had a baseline mild-to-moderate degree of chronic kidney disease (mean estimated glomerular filtration rate 55.0±14.2 mL/min/1.73m2) and mild elevation of glucose levels (132±29 mg/dL). There were no significant changes observed in hemoglobin, white blood cell, and platelet counts; blood urea nitrogen; creatinine; estimated glomerular filtration rate; or glucose levels when comparing baseline values to those obtained on Day 365 after MSC infusion.
| Table 2: Laboratory Variables Before and After MSC Infusion. | |||||
| Laboratory Variables | Preinfusion Day 1 to 7, Mean+SD Median (Range) |
Postinfusion Day 7, Mean+SD Median (Range) |
Postinfusion Day 30, Mean+SD Median (Range) |
Postinfusion, Day 365, Mean+SD Median (Range) |
P Value |
| Hemoglobin, g/dL | 13.2+1.2 13.5 (11.1-14.8) |
13.1+1.2 13.1 (10.9-15.1) |
13.2+1.4 13.5 (10.5-15.1) |
12.7+1.80 13.0 (10.2-15.6) |
.18 |
| WBC count, ×109/L | 6.5+2.1 6.8 (3.3-10.1) |
6.8+1.8 6.7 (3.9-9.38) |
6.4+2 5.6 (3.6-11.1) |
6.4+1.2 6.3 (4.9-7.9) |
.89 |
| Platelet count, ×109/L | 173+39 164 (131-238) |
179+38 179 (130-251) |
176+36 176 (124-242) |
171+21 171 (140-204) |
.86 |
| BUN, mg/dL | 23.7+9.1 22 (11-43) |
21.1+8.7 22 (9-37) |
22.6+9.3 24 (11-41) |
25.4+10.2 22 (14-44) |
.55 |
| Creatinine, mg/dL | 1.3+0.3 1.2 (0.9-1.9) |
1.3+0.3 1.2 (0.9-1.7) |
1.4+0.3 1.4 (0.9-1.9) |
1.58+0.7 1.3 (0.8-3.2) |
.20 |
| GFR, mL/min/1.73m2 | 55.0+14.2 55 (37-79) |
56.4+14.1 57 (40-84) |
50.5+10.9 46 (34-67) |
50.3+17.6 56 (19-72) |
.26 |
| Glucose, mg/dL | 132+30 131 (95-177) |
127+31 135 (89-179) |
141+33 133 (88-197) |
123+28 117 (92-173) |
.40 |
| Abbreviations: BUN- Blood Urea Nitrogen; GFR- Glomerular Filtration Rate; MSC- Mesenchymal Stem Cells; WBC- White Blood Cell. | |||||
Effect of MSC therapy on gas exchange variables
Table 3 displays variables derived from arterial blood gases obtained on Day-7 prior to MSC infusion, and then on Days 7, 30 and 365 after infusion. All patients except 1 had arterial blood gases obtained on room air. One patient from Group 1 used 2 L/minute oxygen flow via a nasal cannula (estimated FIO2=0.28) from baseline and throughout the 1-year follow-up. At baseline, our cohort showed normal ventilation capacity (PaCO2=39.1±3.7 mm Hg) and oxygenation (PaO2=80.2±8.6 mm Hg and SaO2= 95%±1%). The mean baseline FIO2/PaO2 ratio was greater than 350. No significant changes in oxygen requirement, ventilation, or oxygenation occurred within the year after MSC infusion, and ventilation and oxygenation variables were not significantly different a year after infusion compared to baseline values.
| Table 3: Arterial Blood Gases Before and After MSC Infusion. | |||||
| Variable | Preinfusion, Day 7, Mean+SD Median (Range) |
Postinfusion, Day 7, Mean+SD Median (Range) |
Postinfusion Day 30, Mean+SD Median (Range) |
Postinfusion Day 365, Mean+SD Median (Range) |
P Value |
| FIO2 | 0.22+0.02 0.21 (0.21-0.28) |
0.22+0.02 0.21 (0.21-0.28) |
0.22+0.02 0.21 (0.21-0.28) |
0.22+0.02 0.21 (0.21-0.28) |
|
| pH | 7.41+0.03 7.41 (7.34-7.43) |
7.41+0.02 7.41 (7.37-7.45) |
7.42+0.02 7.40 (7.40-7.45) |
7.42+0.03 7.42 (7.40-7.47) |
.31 |
| PaCO2, mm Hg | 39.1+3.9 41 (32-44) |
39+4.9 38 (31-49) |
38.3+3.1 37 (35-45) |
36.4+4.7 36 (29-46) |
.09 |
| PaO2, mm Hg | 80.2+8.6 81 (61-90) |
81.3+10.7 84 (69-96) |
79.1+10.5 80 (65-93) |
81.7+7.9 81 (68-94) |
.61 |
| SaO2, % | 94.9+1.3 95 (92-96) |
94.9+1.9 95 (92-97) |
94.5+1.7 95 (92-96) |
94.9+0.78 95 (94-96) |
>.99 |
| PaO2/FIO2 | 374+62.6 386 (218-429) |
376+54.3 343 (314-457) |
367+68.9 379 (232-443) |
380+58.8 386 (243-448) |
.67 |
| Abbreviations: FIO2- Fraction of Inspired Oxygen Ratio; MSC- Mesenchymal Stem Cells; PaCO2- Partial Pressure of Carbon Dioxide in Arterial Blood; PaO2- Partial Pressure of Oxygen in Arterial Blood; PaO2/FIO2- Partial Pressure of Oxygen in Arterial Blood to Fraction of Inspired Oxygen Ratio; SaO2- Saturation of Oxygen in Arterial Blood | |||||
Pulmonary function testing
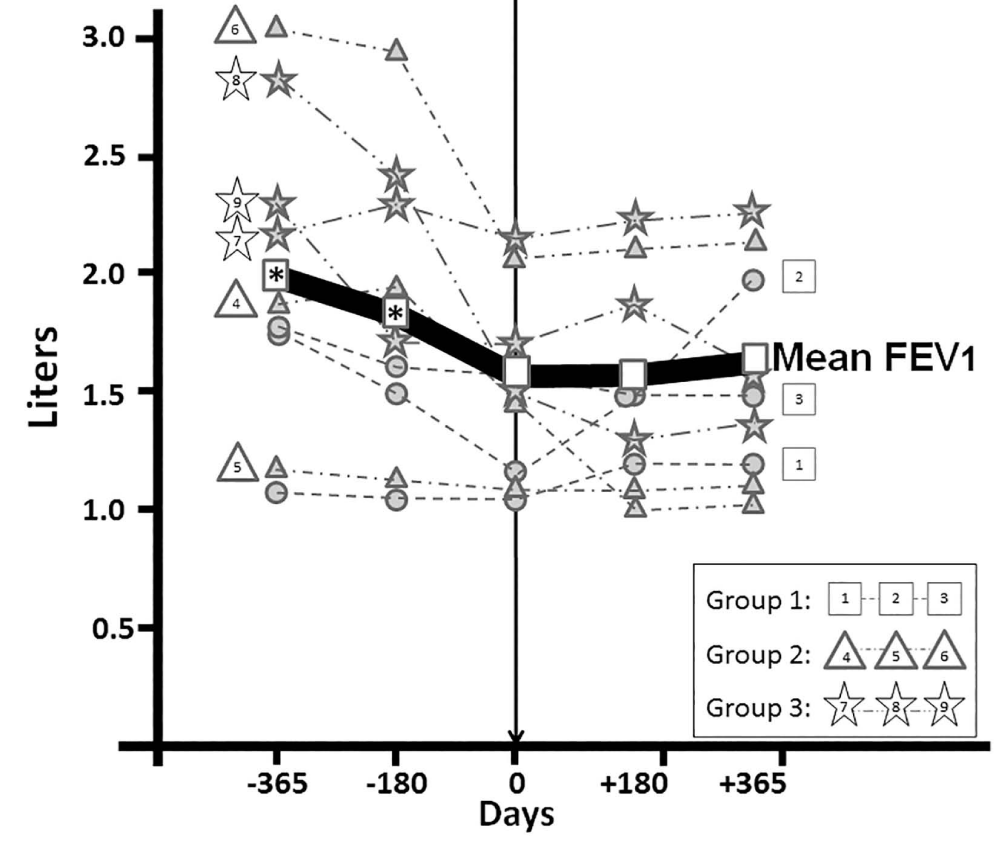
Changes in pulmonary function at Days -365, -180 and 0 prior to infusion of MSCs compared to changes occurring at Days 180 and 365 after MSC therapy are presented in Table 4 and Figure 4. There was a significant drop (P<.05) in mean FVC from Day 365 prior to therapy compared to the mean FVC measured just prior to MSC infusion (Day 0). Likewise, there were significant drops (P<.05) in FEV1 on Days -365 and -180 prior to therapy compared to the FEV1 just before infusion (Day 0). During the year following infusion, the mean FVC and FEV1 for the 9 patients remained not significantly different compared to Day 0. Table: 4 discloses individual changes in FVC and FEV1 before and after therapy, with patients divided into 3 groups according to the dose of MSCs received. The reversal of decline in FVC for this cohort was achieved by 2 patients in Group 1, 1 in Group 2, and 2 in Group 3 (n=5 [55.6%]), showing small increases in FVC compared to Day 0; while 1 patient in Group 1, 2 in Group 2, and 1 in Group 3 (n=4 [44.4%]) continued to have small decreases in FVC compared to Day 0, although at a lesser pace than what was present the year prior to MSC infusion. Similarly, 5 patients (55.6%) had small increases in FEV1 after MSC infusion. The entire group of 9 patients had a mean drop in FVC of 530 mL and a mean drop of 374 mL in FEV1 during the year prior to MSC infusion, while they had a small mean increment of 80 mL and 19 mL in FVC and FEV1, respectively, in the year following MSC infusion suggesting a change in the pattern of lung function following MSC infusion.
Figure 4: Changes in FEV1 the Year Before and After Allogeneic MSC Infusion. Figure shows individual and mean values for FEV1 at Days -365, -180, 0 (just prior to MSC infusion), 180, and 365. * indicates P<0.05 compared to the value obtained at Day 0. FEV1 indicates forced expiratory volume in 1 second; FVC- Forced Vital Capacity; MSC- Mesenchymal Stem Cells
| Table 4: Changes in FVC and FEV1 Before and After MSC Infusion. | ||||||||||
| Dosing Groups |
FVC Preinfusion, Day −365 |
FVC Preinfusion, Day −180 |
FVC Preinfusion, Day 0 |
FVC Postinfusion, Day 180 |
FVC Postinfusion, Day 365 |
FEV1 Preinfusion, Day-365 |
FEV1 Preinfusion, Day-180 |
FEV1 Preinfusion, Day 0 |
FEV1 Postinfusion, Day 180 |
FEV1 Postinfusion, Day 365 |
| Group 1a | 2.02 | 1.94 | 1.78 | 2.05 | 2 | 1.12 | 1.09 | 1.09 | 1.19 | 1.18 |
| 3.72 | 3.12 | 2.19 | 2.84 | 3.4 | 1.71 | 1.53 | 1.16 | 1.53 | 1.95 | |
| 2.47 | 2.37 | 2.29 | 2.22 | 2.11 | 1.72 | 1.6 | 1.58 | 1.54 | 1.53 | |
| Group 2a | 2.31 | 2.37 | 1.88 | 1.58 | 1.54 | 1.83 | 1.92 | 1.5 | 1.01 | 1.05 |
| 2.1 | 1.75 | 1.68 | 1.58 | 1.9 | 1.27 | 1.16 | 1.13 | 1.06 | 1.15 | |
| 5.01 | 4.83 | 4.91 | 5.03 | 4.81 | 3.01 | 2.94 | 2.7 | 2.81 | 2.71 | |
| Group 3a | 3.15 | 3.09 | 2.95 | 2.93 | 3.09 | 2.17 | 2.29 | 2.14 | 2.18 | 2.19 |
| 3.53 | 2.79 | 2.18 | 2.07 | 2.21 | 2.83 | 2.32 | 1.55 | 1.24 | 1.39 | |
| 3.68 | 3.17 | 3.32 | 3.48 | 2.86 | 2.24 | 1.74 | 1.68 | 1.89 | 1.55 | |
| Mean+SD | 3.11+0.98* | 2.83+0.91 | 2.58+1.03 | 2.64+1.1 | 2.66+1.01 | 1.99+0.64* | 1.84+0.6* | 1.61+0.52 | 1.61+0.59 | 1.63+0.55 |
| Median (range) | 3.15 (2.02-5.01) |
2.79 (1.75-4.83) |
2.19 (1.68-4.91) |
2.22 (1.58-5.03) |
2.21 (1.54-4.81) |
1.83 (1.12-3.01) |
1.74 (1.09-2.94) |
1.55 (1.09-2.7) |
1.53 (1.01-2.81) |
1.53 (1.05-2.71) |
| Abbreviations: FEV1- Forced Expiratory Volume in 1 second; FVC- Forced Vital Capacity; MSC- Mesenchymal Stem Cells. Group 1, 1×106 MSC/kg; Group 2, 2×106 MSC/kg; Group 3, 4×106 MSC/kg. *P<.05 compared to Day 0 |
||||||||||
Important clinical events following MSC infusion
There were no major adverse events during, immediately after, or up to a month after MSC infusion in any patient; these observations have been reported by our group in a previous publication [24]. There were a variety of clinical events observed between Day 30 and Day 365 after MSC infusion, reported in Table 5. Since we do not have a control population, it is impossible to define whether or not observed clinical events were related to MSC infusion. Three patients had clinical events following trauma, 2 patients developed skin cancer, and 2 patients developed complications related to atherosclerosis. One patient developed an episode of symptomatic minimal acute rejection with positive transbronchial biopsies 2 months after MSC infusion. At the time of this episode, she developed increased expression of class II human leukocyte antigen antibodies, with a total panel reactive antibody of 83%; 1 of these antibodies (DQ5) was specific to the lung allograft donor. We could not find donor-specific antibodies to the MSC donor in HLA antibody testing. She was treated with pulse corticosteroids and thymoglobulin, with clinical resolution of symptoms. Human leukocyte antigen profile repeated 3 months after treatment was negative.
| Table 5: Important Clinical Events Occurring After MSC Infusion. | |
| Case No. | Clinical Events After MSC Infusion |
| 1 | Herpes labialis, skin cancer, depression, and delirium following spouse's death |
| 2 | Fracture of right leg (fall), DVT and PE after fracture, simvastatin myopathy |
| 3 | Transient ischemic attack |
| 4 | Acute cellular and humoral rejection, positive DSA to MSC donor antigens |
| 5 | Rib fracture from accidental fall |
| 6 | Herniated disk at L5 (epidural injection) |
| 7 | Epstein-Barr virus viremia |
| 8 | Upper respiratory infection, mild depression |
| 9 | Hypertension, skin cancer on face, urosepsis, hydronephrosis (stent placement), CAD (stent placement at right coronary artery) |
| Abbreviations: CAD- Coronary Artery Disease; DSA- Donor-Specific Antibodies; DVT- Deep Vein Thrombosis; L5- Lumbar Vertebra 5; MSC- Mesenchymal Stem Cells; PE- Pulmonary Embolism | |
Discussion
The best method to deliver MSCs to sites of injury has been debated, and whether MSCs need to be in direct contact with target cells or if their effects are mediated by altering the tissue microenvironment via secretion of soluble factors, including growth factors and cytokines [26], or by releasing lipid microvesicles is still being studied [27]. Intravenous administration of MSCs is known to produce the first-pass effect, resulting in cells being trapped by lung tissue [19]. This effect was not a concern in our study because the site of injury was in the lungs; therefore, the intravenous injection of MSCs was expected to deliver the cells to the target tissue. Our intravenously infused MSCs indeed appeared to be trapped in the lungs, as we did not see an increase in circulating MSCs 24 hours after infusion, nor did we observe an increase in circulating MSCs with increasing doses. We did not have the opportunity to measure biochemical markers in bronchoalveolar lavage samples, which could have demonstrated the presence of cells in the alveolar space, or at least, the biochemical impact of their presence. Studies have demonstrated that MSCs respond to chemokine’s, such as SDF-1 [28] and MCF-3 [29]. Inflammatory cytokines, such as tumor necrosis factor α, interferon, and IL-1α, are needed to activate MSCs [16]. Therefore, the infused cells conceivably were chemotactically attracted to the inflamed lung in the setting of moderate obstructive CLAD.
One MSC characteristic is the ability to suppress mitogen-induced lymphocyte (B, NK, and Treg) proliferation in vitro [30] and in vivo [31], although data is limited on systemic antiproliferative effect of MSCs in humans. We observed inconsistent effects of MSC therapy on levels of circulating B and NK cells, but the number of Tregs appeared to increase, nearly doubling by Day 7 in patients who received low or intermediate MSC doses (Groups 1 and 2).
In our study, we observed a significant decrease in proinflammatory cytokines, IL-6 and IL-8, in Group 1, and an increase in tolerogenic cytokine, IL-4, in Groups 1 and 2. In addition, serum analysis of patients in Group 1 showed a significant increase in epidermal growth factor levels, which have an important role in MSC-induced wound healing [32] and tissue regeneration [33]. Analysis of biomarkers suggests that lower doses of MSCs appeared to have more favorable biologic effects. Other clinical studies have suggested similar observations. In a clinical study of ischemic heart failure, the lowest dose was associated with the greatest improvement in left ventricular ejection fraction, and these effects were not present at higher doses [34]. A 32-patient clinical trial treating cases of graft-versus-host disease showed no difference in efficacy or safety among patients receiving 2×106 MSC/kg or 8×106 MSC/kg [35]. These results suggest that lower MSC doses may be as effective as or better than larger doses, and future studies should define whether even lower doses may be as effective, or if there may be specific doses better suited for different clinical conditions.
Our phase 1 trial was designed mostly to define safety and tolerance of administration of MSCs, as reported elsewhere [24]. The extended observations reported here, up to a year after infusion, confirm the absence of anemia, pancytopenia, hyperglycemia, or deterioration in renal function following MSC therapy. The clinical events we observed were not unexpected in an elderly population, many years after lung transplant, subjected to the chronic adverse events related to long-term immunosuppressive therapy. Development of acute rejection and positive donor-specific antibodies to lung allograft donor (not to MSC donor) in 1 patient raises the possibility that this event may have been related to cell therapy, and events like these will need to be defined in larger trials. This particular case was also the only 1 with continued and relentless decline in FEV1 after MSC infusion.
Patients with BOS having mostly progressive airflow obstruction without alveolar disease will have progressive dyspnea on exertion, but not necessarily hypoxemia in early stages. Hypoxemia will eventually appear when BOS becomes severe, with near complete obliteration of airways in larger areas, producing major ventilation-to-perfusion mismatch [36]. Our patients were not hypoxic or hypercapnic prior to MSC therapy, nor did they develop any deterioration in oxygenation or ventilation parameters during the year following MSC infusion. This finding corresponds with the stabilization in lung function observed in the pulmonary function tests. This stabilization of lung function for this cohort was achieved in 5 patients, having a mild improvement in lung volumes and flows following therapy. Another 3 continued to decline in function, but at a lesser rate than prior to MSC infusion. Only 1 continued to have relentless loss in lung function, similar to the steep decline in function observed in the year prior to MSC therapy, despite receiving standard therapy for BOS. Our observations match those reported by Chambers et al. [22], where 8 of 10 patients with moderate or severe obstructive, rapidly progressive CLAD showed a slowing in the rate of decline in FEV1. Their patients received a much larger dose of MSCs compared to our population (total of 8×106 MSC/kg in 4 divided doses of 2×106 MSC/kg). Our study included a less diverse population compared to Chambers et al. [22]; all our patients had BOS-2, had a slower rate of decline in FEV1 and received bone marrow-derived MSCs from a single healthy donor, compared to 5 different donors in the Chambers et al., trial. Despite these differences, both studies showed that, regardless of higher or lower MSC doses, this therapy was safe, well tolerated, and appeared to modify the rate of decline in lung function in some patients who failed standard therapy. Several clinical studies are now reporting the safe administration of cell therapy in patients with an array of lung conditions, such as graft-versus-host disease [37], chronic obstructive pulmonary disease [38] and adult respiratory distress syndrome [39]. To our knowledge, the study from Chambers et al. [22], and our study are the first to recognize a beneficial therapeutic effect in transplant-related BOS.
Our study has limitations. As a phase 1 trial, the patient population is too small to make definite conclusions, and they were further subdivided into 3 groups according to dosage. A significantly more robust protocol of biochemical markers, not only from blood samples, but also from bronchoalveolar lavage samples, is needed to better define the mechanisms by which MSCs exert their effects and the extent of the first pass effect. Subsequent trials with larger numbers of study patients, including control subjects, should give us a better understanding of possible sort and long-term functional as well as adverse events. Detailed human leukocyte antigen screening panels from MSC donors and monitoring patients regularly after infusion of MSCs are also necessary to better understand any clinical effects derived from the development (if any) of donor-specific antibodies.
Conclusion
It is safe and feasible to administer allogeneic bone marrow-derived MSCs to lung transplant recipients with treatment-refractory moderate BOS. Intravenous administration of MSCs may produce a beneficial effect in these patients, by either slowing the rate of functional decline or by actually improving airflow in some patients. These preliminary observations justify a larger, randomized, double-blind study, including control subjects, to better define if indeed there may be a defined therapeutic role of MSC therapy in lung transplant recipients with CLAD.
Acknowledgments
The authors acknowledge the contributions of Francisco Alvarez, Jorge Mallea, and the editorial work of Alison Dowdell.
Funding
This research was supported by grants from the Center for Regenerative Medicine, Mayo Clinic; the Jorge and Leslie Bacardi Fund; PACT (Production Assistance for Cellular Therapy), National Heart, Lung and Blood Institute and a grant by United Therapeutics. The funding agencies had no role in the study design, in the collection, analysis and interpretation of data, on writing the results or in the decision to submit the article for publication.
Presented at The International Society for Heart & Lung Transplantation 36th Annual Meeting and Scientific Sessions, April 27-30, 2016, Washington, DC.
Portions of this manuscript have been published in abstract form: Keller CA, Erasmus DB, Alvarez FG, et-al. Preliminary Report on the Effect of Mesenchymal Stem Cell (MSC) Infusion in Lung Function on Patients with Chronic Allograft Dysfunction (CLAD). J Heart Lung Transplant 2016;35(4 Suppl):S43.
Author contribution
Dr. Keller: Conception and design, Collection and/or assembly of data, Data analysis and interpretation, Manuscript writing, and Final approval of manuscript.
Dr. Gonwa: Conception and design, Data analysis and interpretation, and Final approval of manuscript.
Ms. Russell: Collection and/or assembly of data, Data analysis and interpretation, and Final approval of manuscript.
Mr. Hodge: Data analysis and interpretation, and Final approval of manuscript.
Dr. Erasmus: Collection and/or assembly of data, Data analysis and interpretation, and Final approval of manuscript.
Dr. Zubair: Conception and design, Collection and/or assembly of data, Data analysis and interpretation, Manuscript writing, and Final approval of manuscript.
References
- Weill D, Benden C, Corris PA, Dark JH, Davis RD, et al. A consensus document for the selection of lung transplant candidates. 2014-An update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2015; 34: 1-15. Ref.: https://tinyurl.com/yb6tufvh
- Christie JD, Sager JS, Kimmel SE, Ahya VN, Gaughan C, et al. Impact of primary graft failure on outcomes following lung transplantation. Chest. 2005; 127: 161-165. Ref.: https://tinyurl.com/y9fras99
- Burguete SR, Maselli DJ, Fernandez JF, Levine SM. Lung transplant infection. Respirology. 2013; 18: 22-38. Ref.: https://tinyurl.com/y9wj5gqw
- Martinu T, Chen DF, Palmer SM. Acute rejection and humoral sensitization in lung transplant recipients. Proc Am Thorac Soc. 2009; 6: 54-65. Ref.: https://tinyurl.com/yawkc6gb
- Witt CA, Gaut JP, Yusen RD, Byers DE, Iuppa JA, et al. Acute antibody-mediated rejection after lung transplantation. J Heart Lung Transplant. 2013; 32: 1034-1040. Ref.: https://tinyurl.com/y9gczlm9
- Verleden GM, Raghu G, Meyer KC, Glanville AR, Corris P. A new classification system for chronic lung allograft dysfunction. J Heart Lung Transplant. 2014; 33: 127-133. Ref.: https://tinyurl.com/ydgul2j6
- Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002; 21: 297-310. Ref.: https://tinyurl.com/y84ovdha
- Sato M, Waddell TK, Wagnetz U, Roberts HC, Hwang DM, et al. Restrictive allograft syndrome (RAS): a novel form of chronic lung allograft dysfunction. J Heart Lung Transplant. 2011; 30: 735-742. Ref.: https://tinyurl.com/yaau27zu
- Lin CM, Zamora MR. Update on Bronchiolitis Obliterans Syndrome in Lung Transplantation. Curr Transplant Rep. 2014; 1: 282-289. Ref.: https://tinyurl.com/ybx4294d
- Meyer KC, Raghu G, Verleden GM, Corris PA, Aurora P, et al. An international ISHLT/ATS/ERS clinical practice guideline: diagnosis and management of bronchiolitis obliterans syndrome. Eur Respir J. 2014; 44: 1479-1503. Ref.: https://tinyurl.com/ybk8acgu
- Belperio JA, Weigt SS, Fishbein MC, Lynch JP. Chronic lung allograft rejection: mechanisms and therapy. Proc Am Thorac Soc. 2009; 6: 108-121. Ref.: https://tinyurl.com/yc954hm6
- Corris PA, Ryan VA, Small T, Lordan J, Fisher AJ, et al. A randomised controlled trial of azithromycin therapy in bronchiolitis obliterans syndrome (BOS) post lung transplantation. Thorax. 2015; 70: 442-450. Ref.: https://tinyurl.com/yc82wn6o
- Davis RD Jr, Lau CL, Eubanks S, Messier RH, Hadjiliadis D, et al. Improved lung allograft function after fundoplication in patients with gastroesophageal reflux disease undergoing lung transplantation. J Thorac Cardiovasc Surg. 2003; 125: 533-542. Ref.: https://tinyurl.com/yc4kcfue
- Novick RJ, Stitt LW, Al-Kattan K, Benden C, Dipchand AI, et al. Pulmonary retransplantation: predictors of graft function and survival in 230 patients. Pulmonary Retransplant Registry. Ann Thorac Surg. 1998; 65: 227-234. Ref.: https://tinyurl.com/y88mcnao
- Yusen RD, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first adult lung and heart-lung transplant report--2014; focus theme: retransplantation. J Heart Lung Transplant. 2014; 33: 1009-1024. Ref.: https://tinyurl.com/ydg67cab
- Ren G, Zhang L, Zhao X, Xu G, Zhang Y, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008; 2: 141-150. Ref.: https://tinyurl.com/y8pbzr7b
- Kode JA, Mukherjee S, Joglekar MV, Hardikar AA. Mesenchymal stem cells: immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy. 2009; 11: 377-391. Ref.: https://tinyurl.com/y7a3jha9
- Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, et al. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008; 111: 1327-1333. Ref.: https://tinyurl.com/y735osx8
- Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009; 18: 683-692. Ref.: https://tinyurl.com/ycsx76nv
- Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA. 2003; 100: 8407-8411. Ref.: https://tinyurl.com/ycnna3hj
- Weng JY, Du X, Geng SX, Peng YW, Wang Z, et al. Mesenchymal stem cell as salvage treatment for refractory chronic GVHD. Bone Marrow Transplant. 2010; 45: 1732-1740. Ref.: https://tinyurl.com/ycwo94wq
- Chambers DC, Enever D, Lawrence S, Sturm MJ, Herrmann R, et al. Mesenchymal Stromal Cell Therapy for Chronic Lung Allograft Dysfunction: Results of a First-in-Man Study. Stem Cells Transl Med. 2017; 6: 1152-1157. Ref.: https://tinyurl.com/yaq4q64v
- Keller CA, Erasmus DB, Alvarez FG, Mallea JM, Hurst KE, et al. Preliminary Report on the Effect of Mesenchymal Stem Cell (MSC) Infusion in Lung Function on Patients with Chronic Allograft Dysfunction (CLAD). J Heart Lung Transplant. 2016; 35: S43. Ref.: https://tinyurl.com/ybg72jjy
- Keller CA, Gonwa TA, Hodge DO, Hei DJ, Centanni JM, et al. Feasibility, Safety, and Tolerance of Mesenchymal Stem Cell Therapy for Obstructive Chronic Lung Allograft Dysfunction. Stem Cells Transl Med. 2018; 7: 161-167. Ref.: https://tinyurl.com/ybwn9py6
- Mesenchymal Stem Cell Therapy for Lung Rejection. 2014.
- Rojas M, Xu J, Woods CR, Mora AL, Spears W, et al. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005; 33: 145-152. Ref.: https://tinyurl.com/y7559mfc
- Baglio SR, Pegtel DM, Baldini N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol. 2012; 3: 359. Ref.: https://tinyurl.com/y7utwtsg
- Kitaori T, Ito H, Schwarz EM, Tsutsumi R, Yoshitomi H, et al. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum. 2009; 60: 813-823. Ref.: https://tinyurl.com/y84qm9cq
- Schenk S, Mal N, Finan A, Zhang M, Kiedrowski M, et al. Monocyte chemotactic protein-3 is a myocardial mesenchymal stem cell homing factor. Stem Cells. 2007; 25: 245-251. Ref.: https://tinyurl.com/ycl3lfzh
- Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002; 30: 42-48. Ref.: https://tinyurl.com/y78huexv
- Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005; 106: 1755-1761. Ref.: https://tinyurl.com/yb7fyw9x
- Kim SW, Zhang HZ, Guo L, Kim JM, Kim MH. Amniotic mesenchymal stem cells enhance wound healing in diabetic NOD/SCID mice through high angiogenic and engraftment capabilities. PLoS One. 2012; 7: e41105. Ref.: https://tinyurl.com/jpdqco6
- Khalili S, Liu Y, Kornete M, Nienke R, Shohta K, et al. Mesenchymal stromal cells improve salivary function and reduce lymphocytic infiltrates in mice with Sjogren's-like disease. PLoS One. 2012; 7: e38615. Ref.: https://tinyurl.com/y72dvv9e
- Perin EC, Borow KM, Silva GV, DeMaria AN, Marroquin OC, et al. A Phase II Dose-Escalation Study of Allogeneic Mesenchymal Precursor Cells in Patients With Ischemic or Nonischemic Heart Failure. Circ Res. 2015; 117: 576-584. Ref.: https://tinyurl.com/yambss9v
- Kebriaei P, Isola L, Bahceci E, Holland K, Rowley S, et al. Adult human mesenchymal stem cells added to corticosteroid therapy for the treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009; 15: 804-811. Ref.: https://tinyurl.com/yao3qxue
- Reichenspurner H, Girgis RE, Robbins RC, Conte JV, Nair RV, et al. Obliterative bronchiolitis after lung and heart-lung transplantation. Ann Thorac Surg. 1995; 60: 1845-1853. Ref.: https://tinyurl.com/yab268gm
- Ringden O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006; 81: 1390-1397. Ref.: https://tinyurl.com/y8l2tp2k
- Weiss DJ, Casaburi R, Flannery R, LeRoux-Williams M, Tashkin DP. A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest. 2013; 143: 1590-1598. Ref.: https://tinyurl.com/y89bknpb
- Wilson JG, Liu KD, Zhuo H, Caballero L, McMillan M, et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med. 2015; 3: 24-32. Ref.: https://tinyurl.com/y76yz9p9