Research Article
Enhancing adipose stem cell chondrogenesis: A study on the roles of dexamethasone, transforming growth factor β3 and ascorbate supplements and their combination

Arshan Nazempour##, Chrystal R Quisenberry##, Nehal I Abu-Lail and Bernard J Van Wie*
Gene and Linda Voiland School of Chemical Engineering and Bioengineering, Washington State University, Pullman, WA, 99164-6515, USA
*Address for Correspondence: Dr. Bernard J Van Wie, Gene and Linda Voiland School of Chemical Engineering and Bioengineer-ing, Washington State University, Pullman, Washington, USA, 99164-6515, Tel: 509-335-4103, Fax: 509-335-4806; Email: bvanwie@wsu.edu
Dates: Submitted: 13 June 2017; Approved: 28 July 2017; Published: 31 July 2017
How to cite this article: Nazempour A, Quisenberry CR, Abu-Lail NI, Van Wie BJ. Enhancing adipose stem cell chondrogenesis: A study on the roles of dexamethasone, transforming growth factor β3 and ascorbate supplements and their combination. J Stem Cell Ther Transplant. 2017; 1: 028-051. DOI: 10.29328/journal.jsctt.1001004
Copyright License: © 2017 Wie BJV, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Ascorbate; Atomic force microscopy; Elastic modulus; Cartilage; Chondrogenesis; Dexamethasone; Transforming growth factor β3 and Human-adipose derived stem cells
ABSTRACT
Varied exogenous chondrogenic factors (CFs) are implicated in promoting differentiation of stem cells along a chondrocyte lineage in the field of regenerative tissue engineering for articular cartilage repair. The effects of dexamethasone, transforming growth factor β3 (TGF-β3), ascorbate, and their combinations, on mRNA expression in micromass-cultured human adipose derived stem cells (hADSCs) were investigated as a function of time. Indices include chondrogenic, hypertrophic, angiogenic, fibrogenic and osteogenic markers along with mechanical properties, assessed by atomic force microscopy. Early in the culture, i.e., at day three, no significant differences in mRNA expression of SOX9, aggrecan, lubricin, Col XI, Col X, vascular endothelial growth factor, Col I, and alkaline phosphatase were observed among samples treated with different CFs. However, significant differences in mRNA expression levels of pre-mentioned markers among samples treated with each CF exist when samples were supplied with the CFs for more than three days. A new indexing scheme summing expression of chondrogenic and subtracting non-chondrogenic angiogenic, fibrogenic and osteogenic marker levels shows dexamethasone is the overall leading CF among the factors and their combinations. Based on this scheme, we have projected not only the possible signaling pathways which might be affected by addition of CFs but also hypothetical indexes that may occur upon temporal variation of growth factor regimens.
ABBREVIATIONS
AC: Articular Cartilage; ACAN: Aggrecan; ACTE: Articular Cartilage Tissue Engineering; ALP: Alkaline Phosphatase; ADSC: Adipose derived Mesenchymal Stem Cell; Asc: Ascorbate; BMP: Bone Morphogenic Protein; BMSC: Bone Marrow derived Mesenchymal Stem Cell; CF: Chondrogenic factor; Col: Collagen; Dex: Dexamethasone; DTA: Dex with Asc and TGF-β; DT: Dex with TGF-β3; ECM: Extracellular Matrix; FGF: Fibroblast Growth Factor; GAG: Glycosaminoglycans; GF: Growth Factor; h: human; IGF: Insulin like Growth Factor; MMP: Matrix Metallopeptidase; MSC: Mesenchymal Stem Cell; OA: Osteoarthritis; RUNX2: Runt-related transcription factor 2; SC: Stem Cell; TE: Tissue Engineering; TGF-β: Transforming Growth Factor -β; VEGF: Vascular Endothelial Growth Factor; wrt: with respect to;
INTRODUCTION
The avascularity of articular cartilage (AC) contributes to its inability for self-repair [1]. Damage to AC due to injury or osteoarthritis (OA) results in mechanically substandard fibrocartilage formation [2]. Current clinical therapies for AC repair, including microfracture, abrasion, drilling, osteochondral grafting, and autologous chondrocyte implantation, have limitations such as difficulty in harvesting chondrocytes from donor tissues as well as donor site morbidity [3]. As such, AC tissue engineering (ACTE) is a promising strategy for developing a biological tissue replacement with near native properties.
Because monolayer cultures of chondrocytes leads to chondrocyte dedifferentiation [4], multipotent stem cells, such as bone marrow-derived mesenchymal stem cells (BMSCs) and adipose-derived stem cells (ADSCs) are ideal cellular alternatives for ACTE because they can be supplied in high quantity and have chondrogenic potential [5]. The therapeutic potential of MSCs in OA has been suggested in animal models [6,7]. An intra-articular injection of autologous BMSCs in hyaluronic acid (HA) in surgically induced OA in caprine subjects reduced cartilage degeneration, osteophytic remodeling and subchondrodral sclerosis in 26 weeks [8]. In spontaneous OA in guinea pigs, injection of encapsulated human BMSCs in HA resulted in partial repair with strong collagen type II (Col II) immunostaining in 5 weeks [9]. Autologous BMSC transplantation has been shown effective in patients to the level that Wakitani et al. [10]. treated full thickness AC defects in three patients, who had undergone arthroscopic surgery but still felt pain. Although many studies in the field of ACTE have looked at the use of BMSCs to engineer AC tissues because of their proximity to cartilage surfaces, isolation of ADSCs via liposuction is far less invasive [11], making ADSC use in ACTE of great interest [12,13].
Chondrogenesis is a tightly regulated, multi-step process which is initiated in vivo by mesenchymal stem cell (MSC) condensation. Differentiation of condensed MSCs first encounters the chondroblast phase characterized by cell proliferation and deposition of cartilage-specific proteins such as collagen type II and XI (Col II & Col XI), aggrecan (ACAN) and lubricin. In this phase, a balance between Sex-Determining Region Y Box 9 (SOX9) and runt-related transcription factor 2 (RUNX2) is important to regulate the transition between chondroblasts and osteoblasts. Following this phase, chondrocytes become hypertrophic as they terminally differentiate, characterized by cell enlargement and expression of collagen type X (Col X), matrix metallopeptidase-13 (MMP-13), osteocalcin and vascular endothelial growth factor-A (VEGF-A) [14,15]. Hypertrophic chondrocytes undergo apoptosis and are finally replaced by bone. Cartilage residing at the end of long bones forms when terminal differentiation is blocked [16].
When MSCs are cultured in vitro, addition of growth factors and hormones to the medium can influence the expression of the pre-mentioned components and drive the cells down different differentiation paths. Dexamethasone (Dex), a synthetic glucocorticoid hormone, is a steroid with anti-inflammatory and immunosuppressant properties that has varied effects on the proliferation and differentiation of MSCs. At high concentrations, 10-6 M, Dex suppresses human BMSC (hBMSC) proliferation [17], while at 10-10 M it increases proliferation [18] and enhances expression of chondrogenic markers like ACAN at 10-7 M [19]. Another commonly used additive to induce chondrogenesis in vitro is TGF-β3 which improves expression of chondrogenic markers in both hBMSCs and hADSCs; however, it also induces expression of hypertrophic markers like Col X [20]. Like Dex, the effects of TGF-β3 supplementation on chondrogenesis is dose-dependent [20]. While no significant difference is seen between 10 ng/ml and 25 ng/ml of TGF-β3 in Col II staining or glycosaminoglycan (GAG) content, 2.5 ng/ml of TGF-β3 leads to pellets with limited Col II staining and significantly lower GAG content [21]. Ascorbate (Asc) is thought of as another key nutrient in chondrogenesis. Alginate-encapsulated ADSCs in Asc-containing medium expressed 2.5-fold more proteoglycans than counterparts without Asc [22]. It has also been shown that when Asc is added to the culture medium of ATDC5 cells, a mouse teratocarcinoma that differentiates in a chondrogenic fashion, they significantly up-regulate chondrogenic genes such as SOX9, Col II, and ACAN [23]. Although previous studies provide a general idea of how each supplement will affect chondrogenesis, the exact roles of Dex, TGF-β3 and Asc, individually and synergistically, on temporal ADSC chondrogenesis and construct biomechanical properties remains elusive [20].
Understanding molecular mechanisms and intracellular pathways influenced by exogenous chondrogenic factors (CFs) is key to AC repair. Here we investigate the roles of Dex, TGF-β3, Asc, and Dex plus TGF-β3 (DT), and Dex with TGF-β3 and Asc (DTA) on mechanical properties of engineered tissues and the physiological development sequence of cartilage. We present results on mRNA expression of chondrogenic, hypertrophic, angiogenic, fibrogenic and osteogenic markers in ADSCs cultured in micromass as a function of time. Atomic force microscopy (AFM) nanoindentation measurements were performed on the engineered tissues to quantify the tissue mechanical properties shown to be influenced by extracellular matrix (ECM) deposition [24]. Finally, the structure-function relationship of the engineered tissues were assessed by correlating the gene profiles of key chnodrogenic markers measured via Taqman analyses with the mechanical properties of engineered micromass tissues.
MATERIALS AND METHODS
Cell culture supplies were purchased from Invitrogen-Gibco®, Grand Island, NY, USA unless otherwise specified.
Cell culture
Human ADSCs (hADSCs) isolated from a lipoaspirate tissues from a 33-year-old female, purchased from Invitrogen-Gibco® and as per the Invitrogen website express an ADSC cell surface protein profile including CD29, CD44, CD73, CD90, CD105, CD166 antigens and test negative for CD14, CD31, CD45, Lin1 by flow cytometry. hADSCs were cultured in expansion medium (EM) containing high-glucose Dulbecco’s Modified Eagle’s Medium (HG-DMEM/F12) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100 μg/ml streptomycin (Sigma-Aldrich, St. Louis, MO), and 5 μg/ml gentamicin and maintained under standard conditions (37 °C in a humidified incubator with 5% CO2). Medium was changed three times a week. Upon 80-90% confluency, cells were passaged using GibcoR TrypLE™ Select and used at passage five for the experiments described below.
Micromass culture and differentiation
Among common culture techniques utilized in vitro to induce chondrogenesis, i.e. pellet and micromass, we chose the latter for the following reasons:
1. High density micromass culture, mimicking cellular condensation and the hypoxic environment occurring during in vivo chondrogenesis [25,26], is widely used for the study of in vitro chondrogenesis in stem cells (SCs) [26,27,28].
2. Less than 10% of MSCs cultured in micromass for 21 days are apoptotic [29].
3. When compared to pellet culture, MSC culture in micromass leads to larger, more homogenous cartilage tissues with higher amounts of cartilage specific Col II. MSCs cultured in micromass express less fibrocartilage Col I and hypertrophic Col X markers compared to MSCs cultured in pellets [30].
To generate high density micromass, cells were harvested and resuspended in EM at 1.6×107 cells/ml. Micromass cultures were created by carefully placing a 10 μl droplet cell suspension in the center of each well of a 24-well plastic plate as shown in Figure 1. Cells cultured under standard conditions adhered after 2 hours. A total of 500 μl of fresh EM was then added. After a day, EM was removed and replaced with:
1.Negative control (NC): HG-DMEM/F12 supplemented with 1 mM sodium pyruvate, 2 mM L-glutamine, 5 μg/ml Gentamicin, 1% insulin-transferrin-selenium, 50 μM L-proline (Alfa Aesar, Ward Hill, MA), and 1% penicillin-streptomycin (Sigma-Aldrich).
2. Dexamethasone (Dex): NC+100 nM dexamethasone (Sigma-Aldrich).
3. TGF-β3: NC+10 ng/ml TGF-β3 (PeproTech, Ward Hill, NJ).
4. Ascorbate (Asc): NC+50 μg/ml L-ascorbic acid (Sigma-Aldrich).
5. Dexamethasone and TGF-β3 combined: NC+100 nM Dex+10 ng/ml TGF-β3.
6. Dexamethasone, TGF-β3 and ascorbate combined (DTA): NC+100 nM Dex+50 μg/ml Asc+10 ng/ml TGF-β3.
Micromass samples were maintained under standard conditions, at 37 °C in a humidified incubator with 5% CO2, throughout the experiment. We chose to study the effect of each supplement in a serum free microenvironment to avoid lot-to-lot variability associated with bovine serum [31] and to minimize the effects of undefined components of bovine serum on chondrogenesis.
RNA isolation and analysis
Quantitative real time polymerase chain reaction (qRT-PCR) was used to quantify gene expression. Briefly, total RNA was isolated from four pooled micromass samples with TRIzol. For each treatment time point, three replicates (total of twelve micromass samples) were used for qRT-PCR. Chloroform was utilized for phase separation. Total mRNA (up to 2.5 μg) was reverse-transcribed into cDNA using SuperScript® VILO™ Master Mix. cDNA was amplified with the TaqMan® Gene Expression Master Mix (Applied Biosystems by Life Technologies, Grand Island, NY, USA) on an ABI 7900HT Sequence Detection System (Applied Biosystems) and probes specific for GAPDH, a housekeeping gene that has served as a baseline in several studies on chondrocyte-progenitor interaction and MSC-chondrocyte co-culture [32-37], sex-determining region Y (SRY)-box 9 (SOX9), ACAN, Lubricin, Col XI, Col X, VEGF-A, Col I, alkaline phosphatase (ALP), and osterix were used. Known primer sequences for these species appear in Table 1, and where primer sequences are proprietary, the company name and catalogue number are given. The relative gene expression was calculated using the ∆∆CT method and fold differences were determined using the expression [38], where hASC values prior to the differentiation assays were considered as the reference or control. Micromass was collected at days 3, 7, 14, and 25.
| Table 1: Primer sequences or source information. | |||
| Gene | Probe Sequence | Forward Primer | Reverse Primer |
| hGAPDH | TCAACAGCGACACCCACTCCTC | CCAGGTGGTCTCCTCTGACT | GCTTGACAAAGTGGTCGTTGA |
| hLubricin | GCTCCAACTACTCCTGAGACA | CCTCCTCCAACCACTTCAGAGGTC | GGTAGGCTCCTTGGTGGTA |
| Gene | Company | Catalog No. | |
| hSOX9 | ThermoFisher SCIENTIFIC | Hs00165814_m1 | |
| hACAN | ThermoFisher SCIENTIFIC | Hs01048726_m1 | |
| hCol XI | ThermoFisher SCIENTIFIC | Hs01097678_m1 | |
| hCol X | ThermoFisher SCIENTIFIC | Hs00166657_m1 | |
| hVEGF-A | ThermoFisher SCIENTIFIC | Hs00900055_m1 | |
| hCol I | ThermoFisher SCIENTIFIC | Hs00164004_m1 | |
| hALP | ThermoFisher SCIENTIFIC | Hs01029144_m1 | |
| hOsterix | ThermoFisher SCIENTIFIC | Hs01866874_s1 | |
Mechanical properties of differentiated micromasses
For analysis of mechanical properties, micromass samples were collected at days 3, 7 and 25. Glass slides with adhered micromass samples were removed from the wells and washed with phosphate buffered saline (PBS). The glass underside was glued to a stainless steel disk and mounted onto the AFM sample stage. AFM force-indentation measurements were performed with a PicoForce scanning probe microscope with a Nanoscope IIIa controller and extender module (Bruker AXS Inc., Santa Barbara, CA). To measure the aggregate elastic properties, we used colloidal probes with a manufacturer spring constant of 0.08 N/m and average deflection sensitivity of 81.1±19.0 nm/V (n=3). Prior to force measurements, the actual spring constant of each cantilever was determined using the power spectral density of the thermal noise fluctuations in PBS [39]. Each micromass submerged in PBS was scanned in contact mode over an area of 100 μm2 with a 1 Hz scan rate. Three areas per treatment group were scanned with equally spaced indentation points following a 16×16 grid with an 8 nN trigger threshold of applied load.
Hertz model for Young’s modulus quantification
To quantify the Young’s modulus, AFM approach position-deflection data files were converted to force-indentation data as described previously [40] and fit to the Hertz contact model as described in Equation 1:
Where:
F = applied force
Eγ = Young’s modulus
R = colloidal probe tip curvature radius (constant at 2.6 μm)
ν = Poisson’s ratio (constant at 0.5 based on literature) (16, 65, 95).
δ = indentation depth.
The Young’s moduli were compiled and averaged for each treatment group. Despite limitations in the Hertz model, it is appropriate for our purposes because, the sample surface is continuous and non-conforming, the radius of the contacting bodies is large compared to the contact area so surfaces can be approximated as elastic half spaces [41], and substrate effects can be ignored because the indentation depth (~900 nm) is much smaller than sample thickness (>1 mm height) [42-44]. Frictional forces, probe and sample geometries can be neglected as described previously [45,46]. Although cartilage is a viscoelastic material, we use the Hertz model to fit only the elastic portion of the AFM approach force-distance curve.
Statistical analysis
Statistical analysis was performed using Prism 7 (GraphPad Software, La Jolla, CA) software. Two-way analysis of variance (ANOVA) was used with the Dunn test to determine significant differences between treatment groups (p<0.05). In many instances, significant differences are present and reveal important findings as will be discussed.
RESULTS
Expression of chondrogenic markers
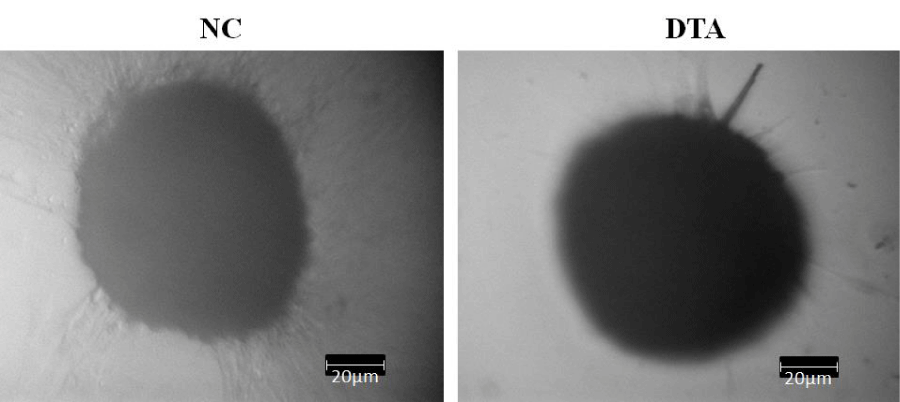
We observed that micromass samples of hADSCs cultured under all conditions, i.e. NC, Dex, TGF-β3, Asc, DT and DTA, condensed and formed nodules as early as day 3. As chondrogenesis in vivo initiates with MSC condensation, we interpret nodule formation as an indicator of micromass sample differentiation toward chondrocytes. Shown in Figure 2 are exemplary photos of NC and DTA micromass samples under light microscopy at 10X where individual cells are no longer visible, as the extracellular matrix is formed around adherent cells. All other cultures with CF additives showed the same condensation phenomenon.
Figure 2: Example images of ADSC condensation and nodule formation as early as day 3 under light microscopy at 10X. NC: HG-DMEM/F12 supplemented with 1 mM sodium pyruvate, 2 mM L-glutamine, 5 μg/ml Gentamicin, 1% insulin-transferrin-selenium, 50 μM L-proline, and 1% penicillin-streptomycin; DTA: NC+100 nM Dex+50 μg/ml Asc+10 ng/ml TGF-β3.
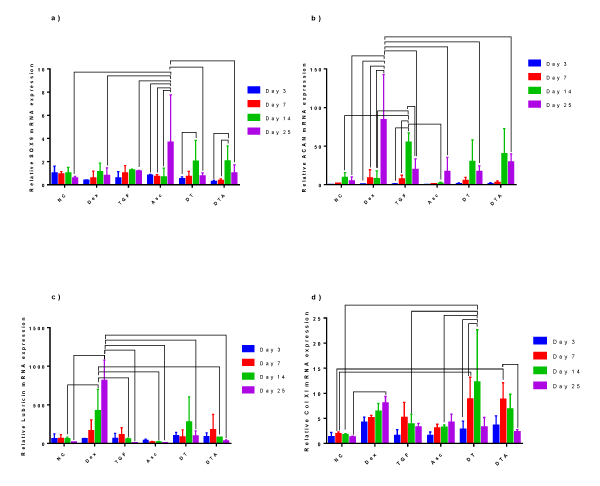
SOX9 expression levels, ratioed to day 0 levels for unstimulated ADSCs, remained near or slightly below 1 till day 7 as shown in Figure 3a. At day 14, a statistically significant increase was observed in SOX9 expression for DT and DTA, and at day 25 for Asc.
Figure 3: A grouped bar graph of the mRNA expression of the chondrogenic markers, a) Sox9, b) ACAN, c) lubricin, and d) Col XI at days 3, 7, 14 and 25. Error bars indicate standard deviation and connected treatments are significantly different. The amount of target normalized to housekeeping gene, GAPDH, and hASC values obtained prior to differentiation assays at day 0 were considered as the reference. NC: HG-DMEM/F12 supplemented with 1 mM sodium pyruvate, 2 mM L-glutamine, 5 μg/ml Gentamicin, 1% insulin-transferrin-selenium, 50 μM L-proline, and 1% penicillin-streptomycin; Dex: NC+100 nM dexamethasone; TGF: NC+10 ng/ml TGF-β3; Asc: NC+50 μg/ml L-ascorbic acid; DT: NC+100 nM Dex+10 ng/ml TGF-β3; and DTA: NC+100 nM Dex+50 μg/ml Asc+10 ng/ml TGF-β3.
ACAN mRNA expression remained close to that of day 0 unstimulated ADSCs increasing by day 14 with statistically significant maxima at day 14 for TGF-β3-treated samples, figure 3b. In NC, TGF-β3, DT and DTA samples, ACAN expression decreased by day 25, and always remained above its expression at day 3. Dex and Asc samples showed generally a continuous increase in ACAN over time with the highest ACAN expression observed in Dex-treated samples at day 25. To the best of our knowledge, Asc’s role on ACAN expression of ADSCs as a function of time has not been studied. Here, we observed Asc supplementation did not significantly affect ACAN expression over the culture period.
Lubricin expression, Figure 3c, was upregulated as early as day 3 with 38- to 97-fold induction compared to day 0 untreated ADSCs. At day 14, Dex-treated samples expressed significantly more lubricin compared to NC, TGF-β3, Asc and DTA samples. Lubricin mRNA expression continuously increased in Dex-treated samples and Dex supplementation caused the highest upregulation of lubricin compared to all other treatments at day 25 with an 814-fold induction over day 0 unstimulated ADSCs. Although not significant, our results show that lubricin has higher expression in TGF-β3-treated samples compared to Asc-treated samples with 1.6-, 6.1-, 3.2- and 1.2-fold increases at day 3, 7, 14 and 25, respectively. It is important to note that the individual role of Dex on lubricin expression is not well documented, yet, here, for the first time, we observed significant upregulation.
Col XI, Figure 3d, increased significantly in expression levels in DT- and DTA-treated samples compared to NC-treated samples at day 7. Interestingly, even though there were no significant differences between NC-, Dex- and TGF-β3-treated samples at day 14, when ADSCs were supplied with a combination of Dex and TGF-β3 for 14 days, cells expressed significantly more Col XI than NC samples and the highest expression of Col XI was achieved. Thus, we conclude that Dex and TGF-β synergistically increase Col XI expression. The effects of Asc alone or in combination with Dex and TGF-β on Col XI expression was studied by Derfoul et al. [19], who cultured hBMSCs in pellets. After three weeks, similar to our day 25 results, they observed similar levels of Col XI expression in both Asc- and DTA-treated samples.
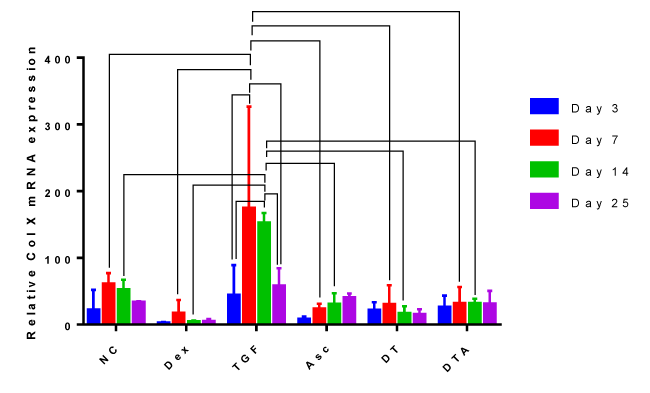
Expression of the hypertrophic marker Col X
Col X mRNA expression, Figure 4, increased by 1- to 3-fold in NC-treated samples compared to Dex-, Asc-, DT-, and DTA-treated samples at all-time points; however, no significant differences between groups were observed. Interestingly, Col X expression in NC-, Dex-, Asc-, DT- and DTA-treated samples did not vary significantly as a function of time. However, in TGF-β3-treated samples, Col X expression was significantly upregulated at day 7 compared to day 3. Even though Col X was expressed more in TGF-β3-treated samples than in any other CF treatment at any time point, it was significantly higher at days 7 and 14 compared to other treatments.
Figure 4: A grouped bar graph of the mRNA expression of the hypertrophic marker, Col X, at days 3, 7, 14, and 25. Error bars indicate standard deviation and connected treatments are significantly different. The amount of target normalized to housekeeping gene, GAPDH, and hASC values obtained prior to differentiation assays at day 0 were considered as the reference. NC: HG-DMEM/F12 supplemented with 1 mM sodium pyruvate, 2 mM L-glutamine, 5 μg/ml Gentamicin, 1% insulin-transferrin-selenium, 50 μM L-proline, and 1% penicillin-streptomycin; Dex: NC+100 nM dexamethasone; TGF: NC+10 ng/ml TGF-β3; Asc: NC+50 μg/ml L-ascorbic acid; DT: NC+100 nM Dex+10 ng/ml TGF-β3; DTA: NC+100 nM Dex+50 μg/ml Asc+10 ng/ml TGF-β3.
We believe this study is the first to report the individual role of Dex on Col X expression by hADSCs as a function of time. As can be seen from Figure 4, Dex-treated samples had the least Col X expression compared to all other treatments at any given time whereas TGF-β3-treated samples exhibited the highest level of Col X expression.
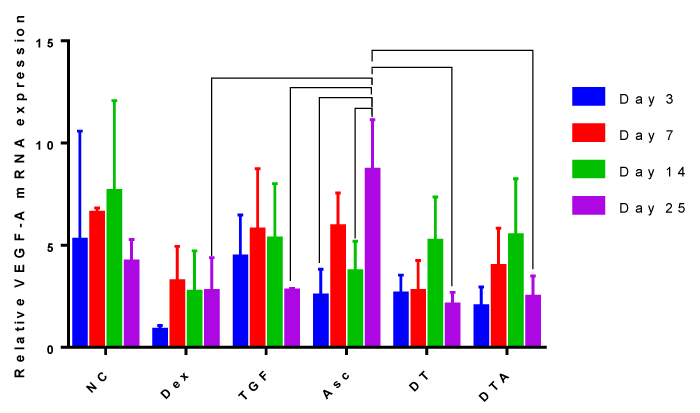
Expression of the angiogenic marker, VEGF-A
Despite high expression of VEGF-A in NC samples, Figure 5, significant differences were only observed in day 25 cultures of Asc-treated samples. This means that all other treatment groups did not vary significantly as a function of time.
Figure 5: A grouped bar graph of the mRNA expression of the angiogenic marker, VEGF-A, at days 3, 7, 14 and 25. Error bars indicate standard deviation and connected treatments are significantly different. The amount of target normalized to housekeeping gene, GAPDH, and hASC values obtained prior to differentiation assays at day 0 were considered as the reference. NC: HG-DMEM/F12 supplemented with mM sodium pyruvate, 2 mM L-glutamine, 5 μg/ml Gentamicin, 1% insulin-transferrin-selenium, 50 μM L-proline, and 1% penicillin-streptomycin; Dex: NC+100 nM dexamethasone; TGF: NC+10 ng/ml TGF-β3; Asc: NC+50 μg/ml L-ascorbic acid; DT: NC+100 nM Dex+10 ng/ml TGF-β3; DTA: NC+100 nM Dex+50 μg/ml Asc+10 ng/ml TGF-β3.
Expression of the fibrogenic marker, Col I
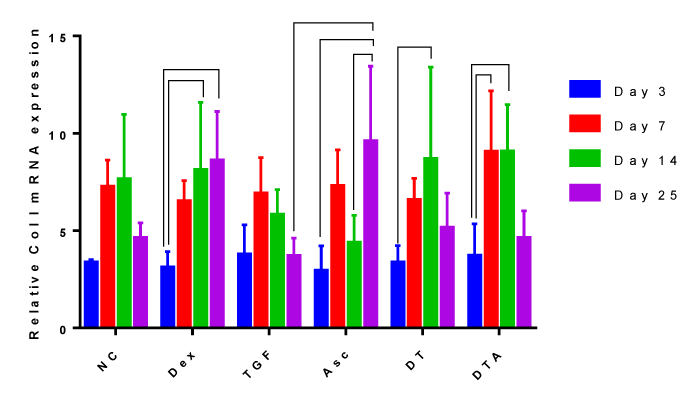
The addition of CFs did not significantly change Col I mRNA expression compared to the NC, Figure 6. Culture duration did not significantly change Col I mRNA expression in NC and TGF-β3 samples. Here, we observed continuous Dex supplementation results in continuously increasing Col I induction during culture and significantly increased Col I by by day 25 for Asc, day 14 for DT and days 7 and 14 for DT and DTA.
Figure 6: A grouped bar graph of the mRNA expression of the fibrogenic marker, Col I, at days 3, 7, 14 and 25. Error bars indicate standard deviation and connected treatments are significantly different. The amount of target normalized to housekeeping gene, GAPDH, and hASC values obtained prior to differentiation assays at day 0 were considered as the reference. NC: HG-DMEM/F12 supplemented with 1 mM sodium pyruvate, 2 mM L-glutamine, 5 μg/ml Gentamicin, 1% insulin-transferrin-selenium, 50 μM L-proline, and 1% penicillin-streptomycin; Dex: NC+100 nM dexamethasone; TGF: NC+10 ng/ml TGF-β3; Asc: NC+50 μg/ml L-ascorbic acid; DT: NC+100 nM Dex+10 ng/ml TGF-β3; DTA: NC+100 nM Dex+50 μg/ml Asc+10 ng/ml TGF-β3.
Expression of osteogenic markers
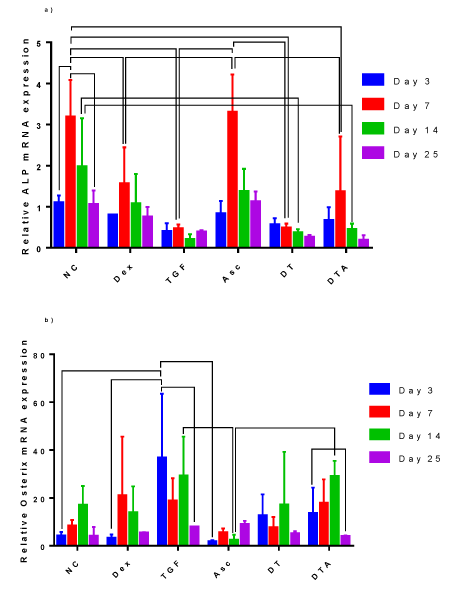
ALP mRNA, Figure 7a, shows a peak for all treatments except DT at day 7. NC- and Asc-treated samples expressed statistically higher ALP, at least by 2-fold, than all other treatments at day 7. To a lesser extent, Day 14 NC cultures were statistically higher, at least 4.44-fold, than cultures containing TGF-β3 (TGF-β3, DT and DTA). ALP expression in Dex, TGF-β3, DT and DTA treatments did not vary significantly with time.
Figure 7: A grouped bar graph of the mRNA expression of the osteogenic markers, a) ALP, b) osterix, at days 3, 7, 14 and 25. Error bars indicate standard deviation and connected treatments are significantly different. The amount of target normalized to housekeeping gene, GAPDH, and hASC values obtained prior to differentiation assays at day 0 were considered as the reference. NC: HG-DMEM/F12 supplemented with 1 mM sodium pyruvate, 2 mM L-glutamine, 5 μg/ml Gentamicin, 1% insulin-transferrin-selenium, 50 μM L-proline, and 1% penicillin-streptomycin; Dex: NC+100 nM dexamethasone; TGF: NC+10 ng/ml TGF-β3; Asc: NC+50 μg/ml L-ascorbic acid; DT: NC+100 nM Dex+10 ng/ml TGF-β3; DTA: NC +10 nM Dex+50 μg/ml Asc+10 ng/ml TGF-β3.
Osterix mRNA expression, Figure 7b, was significantly up-regulated in TGF-β3-treated samples by 37-fold compared to day 0 unstimulated ADSCs early in culture, day 3, which was significantly higher than NC-, Dex- and Asc-treated samples at the same time point. Interestingly, osterix mRNA expression was higher in TGF-β3 samples compared to other treatments.
Mechanics of micromass tissues as assessed by AFM force-indentation measurements
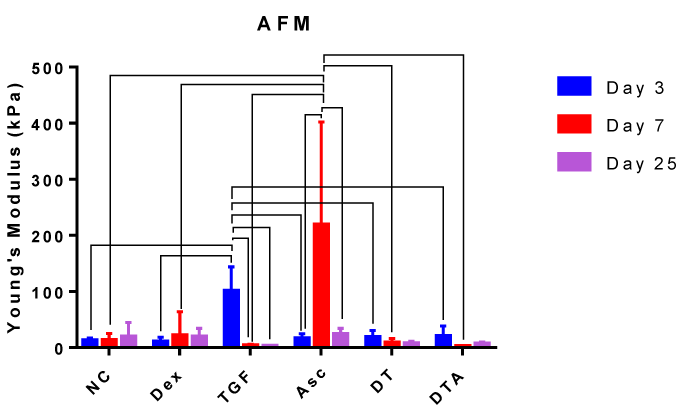
Nanoindentation by AFM was performed and fit to the Hertz model of contact mechanics to characterize the elasticity of micromass tissues grown with different medium supplements at days 3, 7 and 25 as shown in Figure 8. The highest elastic moduli were observed at day 3 with TGF-β3 and at day 7 with Asc. Day 3 TGF-β3 treatment was at least 25-fold higher than any other TGF-β3 treatments and 5-fold higher than other treatments at day 3. Day 7 Asc treatment was at least 9.2-fold and 10-fold higher compared to any other Asc treatment and compared to other treatments at day 7, respectively. At days 7 and 25, a decrease in elastic modulus compared to NC was observed for all treatments containing TGF-β3 (TGF-β3, DT and DTA). As will be discussed later, an optimal elastic modulus should be high enough to support loads, however not as high as values associated with bone formation.
Figure 8: Bar graph representing the elastic modulus for each treatment group at day 3, 7 and 25 as indicated. Error bars indicate standard error of mean. Error bars indicate standard deviation and connected treatments are significantly different. The amount of target normalized to housekeeping gene, GAPDH, and hASC values obtained prior to differentiation assays at day 0 were considered as the reference. NC: HG-DMEM/F12 supplemented with 1 mM sodium pyruvate, 2 mM L-glutamine, 5 μg/ml Gentamicin, 1% insulin-transferrin-selenium, 50 μM L-proline, and 1% penicillin-streptomycin; Dex: NC+100nM dexamethasone; TGF: NC+10 ng/ml TGF-β3; Asc: NC+50 μg/ml L-ascorbic acid; DT: NC+100 nM Dex+10 ng/ml TGF-β3; DTA: NC+100 nM Dex+50 μg/ml Asc+10 ng/ml TGF-β3.
DISCUSSION AND FUTURE STUDIES
Our results and discussion are focused on statistically significant differences between marker expression levels or Young’s moduli. Large standard deviations are not necessarily due to small sample sizes. Though qRT-PCR is one of the most sensitive quantitative methods [47], large error bars and lack of statistical differences often arise because of tissue heterogeneities [48,49], due to or resulting from segregation from precursors, embryological origin, vascularity, and hypertrophy [50]. To compensate, our three data points were comprised of four pooled cultures. Our Standard deviations or mRNA levels are as large and sometimes smaller than values in Acharya et al., study (two-three replicates) [51], Bruce et al., (two-six replicates) [52], and even studies with larger sample sizes of N ≥ 25 [53,54]. Such variations are also due to mixtures of differentiated and undifferentiated cells and a continuum of mRNA marker expression [23,55-57]. Hence, even with large standard deviations, mean differences infer the influence of one additive over another. A reader may also infer trending where statistical differences are lacking, or use this information as a basis for further study. Differences between our data and the literature may be due to differences in cell type and density, culture and handling techniques, growth factor concentrations, sampling times, and biological diversity.
The majority of previous studies evaluated the effects of common CFs on ADSC chondrogenic potential for a limited number of chondrogenic, and non-chondrogenic markers and typically do so at the culture end point [22,58,59]. Here we offer more comprehensive data measuring effects of Dex, TGF-β3, Asc and selected combinations, DT and DTA, and do so temporally for chondrogenic, hypertrophic, angiogenic, fibrogenic and osteogenic markers.
We start our discussion with SOX9, a transcriptional regulator of chondrogenic differentiation [60]. We consider SOX9 as an appropriate chondrogenic marker as many other researchers in the field [61-63] have done. Kawakami et al. [64], named SOX9 as the key intracellular molecule in chondrogenesis; Bell et al. [65], correlated the expression of SOX9 as a marker for expression of Col II; and Zhao et al. suggested SOX9 is needed for expression of the chondrocyte phenotype. Limited literature shows SOX9 upregulated 1.25-fold [66], for hADSCs with TGF-β3 at day 28 consistent with our 1.2-fold increase at day 25, 2-fold for ATDC5 micromass with Asc at day 21 [23], consistent with the 3.9-fold increase we observed at day 25, and 2-fold for bovine BMSC micromass with Dex at day 14 [67], consistent with our 1.1-fold increase at day 14. However, the BMSC study shows no significant difference between Dex and DT, similar to our comparative observations between Dex and DT though both our study and the BMSC study show that addition of Dex enhances TGF-β induced SOX9 expression at day 14.
ACAN, the most abundant proteoglycan in AC, interacts with water to retain high osmotic pressures allowing AC to resist compressive loads [20]. Limited literature on single time points shows an ACAN increase of 100-fold with DT supplementation for ADSCs encapsulated in alginate beads at day 14 [68], whereas we observed a 35-fold induction at day 14, 25- and 2.5-fold for hBMSCs either in pellets [54] or encapsulated in polyethylene glycol diacrylate [69], at day 21 whereas we observed a 3.5-fold induction in ACAN expression in DT-treated samples at day 25. The difference between fold changes may be attributable to differences in cell types, cell density, culture and handling techniques.
Lubricin, a surface-active mucinous glycoprotein, provides articular joint lubrication [70] and prevents chondrocyte apoptosis [71]. Based on our findings, we suggest the use of Dex as a factor to increase lubricin mRNA expression. Literature shows lubricin upregulated 2- and 10-fold for monolayer-cultured bovine articular chondrocytes supplemented with Asc and TGF-β1 for 24 hours [72], respectively. Collagen fibrils are essential for AC structural stabilization [73] and Col XI, while abundant in AC [74] is necessary for skeletal development [75]. Similar to what we observed here, Shintani et al. [67] observed no significant differences in Col XI expression between NC-, Dex- and TGF-β1-treated micromass samples of bovine BMSCs but found significant differences between NC and DT at day 14.
Non-chondrogenic markers indicate unwanted differentiation genotypes. Collagen X, associated with terminally differentiating chondrocytes, is synthesized by hypertrophic chondrocytes [14] and facilitates ECM calcification and mineralization [76]. Consistent with our results at day 25, high levels of Col X mRNA were detected in ASC pellets supplemented with TGF-β3 for 28 days [66] as TGF-β induces hypertrophy. Similar to our results at day 14, significantly more Col X was detected in hBMSC pellets treated with TGF-β1 for 16 days than Asc-treated counterparts [77]. Thus, we conclude that TGF-β3 supplementation strongly directs ADSC differentiation toward hypertrophic lineages. VEGF-A, the major angiogenic factor [78], induces MMP expression in arthritic cartilage and OA [79,80]. Our results for hADSCs in micromass at day 7 are consistent with results obtained by Lee et al. [81] on a 5-day monolayer of rat ADSCs which showed Dex, Asc and TGF-β supplementation down-regulate VEGF-A expression compared to NC. Both studies indicate Dex supplementation results in the least expression of VEGF-A.
Collagen I, the primary collagen in skin, bone, adipose tissue, and fibrocartilage, but not articular cartilage is upregulated in vitro in chondrocyte monolayers on de-differentiation [20] and in vivo in the OA phenotype [82]. Limited literature on single time points shows a 2-fold down-regulation of Col I in alginate-encapsulated ADSCs with Dex at day 7 consistent with our 1.2-fold reduction [83]; a 2-fold upregulation of Col I for rat ADSCs encapsulated in a collagen sponge with TGF-β at day 14 [84] consistent with the 6-fold increase we observed for hADSCs at day 14; no significant difference between Asc and DTA for hBMSCs in pellets at day 16 [77] similar to our comparative observations between Asc and DTA for hASCs at day 14 [83].
ALP, a membrane-bound metalloenzyme, catalyzes phosphomonoester hydrolysis, indicates hypertrophy [85,86] and is elevated in OA and rheumatoid arthritis [87]. Our results indicate that TGF-β3 samples expressed less ALP compared to Dex- and Asc-treated samples at all-time points. This seems valid since Dex and Asc are also used in osteogenic differentiation [85,88-90]. Osterix, a transcription factor for bone formation [91,92], regulates calcification and degradation of chondrogenic matrices through MMP-13 is assumed to be involved in OA [93]. High osterix expression in TGF-β3 samples in our experiments is consistent with literature in which 12-fold increases in 28-day old ADSC pellets was observed [66]. These results suggest that TGF-β3 may be directing ADSCs toward osteogenesis. Here for the first time, we explored the roles of NC, Dex, Asc, DT and DTA supplementation on osterix expression by hADSCs as a function of time and find that NC-, Dex-, Asc-, and DT-treated samples do not change over time.
To enhance the discussion, we report a temporal collective index for CF influence on hADSC chondrogenesis as defined in Equation 3:
where (chondrogenic markers)@day i represents the sum of relative mRNA expression of chondrogenic markers at day i. Considering we have measured and presented mRNA expression for what we consider four (4) chondrogenic factors and five (5) non-chondrogenic markers, we assign 25% to each of the chondrogenic factors (4*25% =100%) and 20% to each of the non-chondrogenic markers (5*20%=100). We allocate equal weights to each factor; others may choose to weigh certain factors more heavily if for example one factor is found mechanistically to be more important to chondrogenesis. Some may argue that a more appropriate index is Col II rather than SOX9 as SOX9 regulates chondrogenesis and does not itself code for the production of chondrogenic proteins. Nevertheless, in this study we have provided rationale for our choice of SOX9 and Col XI, and more importantly demonstrate a new way of looking at collective sets of mRNA data that can be tailored to the markers of interest in any study. To that end positive indices suggest larger weighted contributions of chondrogenic versus non-chondrogenic markers whereas negative indices suggest non-chondrogenic markers outweigh the benefit of chondrogenic markers.
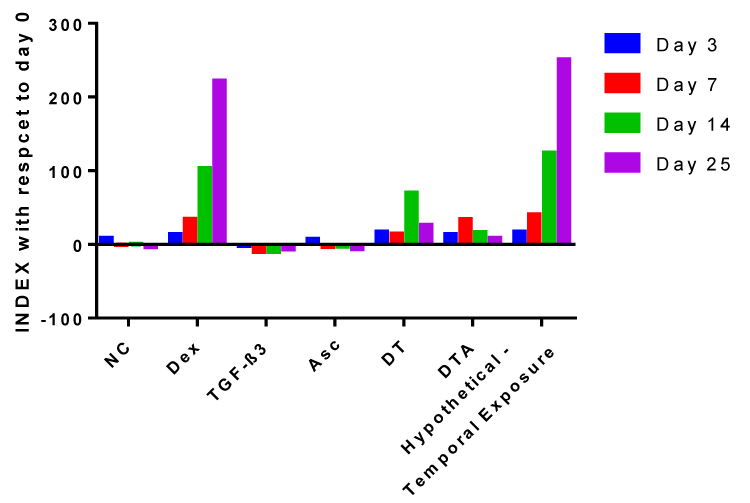
NC-treated samples, as shown in Figure 9, have negative indices for all time points except day 3. This agrees with the results presented in Figures 3-6, describing the upregulation of all non-chondrogenic markers at days 7, 14 and 25 with NC treatment compared to unstimulated ADSCs at day 0. The negative impact of NC culture can be explained by the fact that its base contains HG-DMEM and literature shows that a HG culture enhances adipogenesis of hADSCs [94] and mouse BMSCs [95] compared to low-glucose medium. Furthermore, hBMSCs are less chondrogenic when expanded in HG compared to a low-glucose medium [96]. Given these results, future studies investigating the effects of different glucose concentrations in both expansion and differentiation medium on hADSC chondrogenesis would be of interest.
Dex-treated samples, as shown in figure 9, have positive indices at every time point which is consistent with the literature [97-99] where Stewart et al. [49], reported 2.1- and 2.5-fold increases in DNA and Col II, respectively, in Dex-supplemented equine BMSC pellets after 14 days compared to controls.
Figure 9: Indices as defined by Equation 3 with respect to unstimulated ADSCs at day 0. We emphasize the novelty of the chondrogenic index we have introduced as a way of combining all mRNA analyses. In addition, temporal vacillations in expression patterns allow us to project hypothetical indices that may occur upon switching to a new CF or CF combination that is more effective during a different time period.
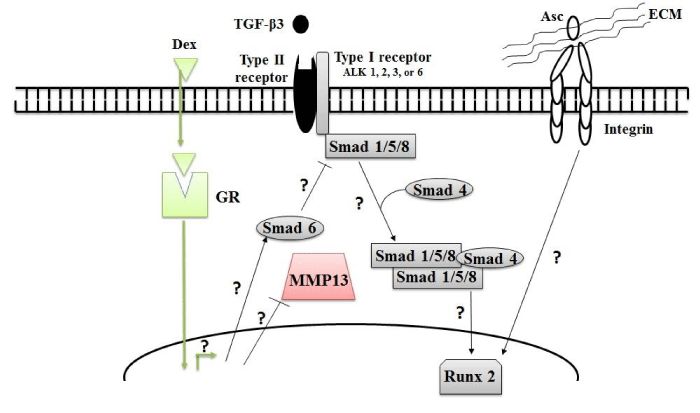
In Figure 10, a cartoon schematic is used to illustrate the prospective influences of the CFs used in this study. Regarding Dex the exact mechanism of action during chondrogenesis of ADSCs is not well understood. However, reports show Dex acts to suppress the expression of interleukin 1β, an inflammatory cytokine identified in osteoarthritic cartilage [100] that induces expression of catabolic factors such as MMPs and aggrecanases [101,102] in multiple cell types [48,103,104]. We suspect suppression of interleukin 1β partially accounts for the effects of Dex on hADSC differentiation. Nonetheless, we recommend future studies to clarify the underlying molecular mechanism of Dex’s anabolic functions on hADSCs with the use of whole genome sequencing.
Figure 10: Possible signaling pathways which might be affected by addition of commonly used CFs. ALK: activin receptor-like kinase, GR: glucocorticoid receptor, MMP13: matrix metalloproteinase and Runx2: runt-related transcription factor 2.
TGF-β3 is known to play a profound role in all phases of chondrogenesis, [105,106] but its influence on ADSC differentiation to chondrocytes or osteocytes is greatly debated. Our data show negative indices at all-time points indicating that TGF-β3 favors osteogenesis over chondrogenesis. This trend is congruent with previous studies showing that TGF-β supplementation induces both chondrogenic and non-chondrogenic expression. Cooke et al. [54] observed a 25-fold induction in ACAN but also 57- and 5-fold increases in Col X and MMP-13 expression, respectively, with 21-day TGF-β supplementation in hBMSC pellet cultures compared to controls. Moreover, Rich et al. [66], detected high levels of Col X and osterix mRNA in hADSC pellets supplemented with TGF-β3 for 28 days.
As shown in Figure 10 TGF-βs transduce their signals by first binding to TGF-β type II receptors. The type I receptor is then recruited to form a heterotetramer and phosphorylate receptor-Smads [107]. Some TGF-β superfamily type I receptors, activin receptor-like kinase (ALK)1, 2, 3 and 6 signals via the Smad1, 5 or 8 pathway, while ALK4, 5 and 7 signal by phosphorylating Smad2 or 3 [108]. The Smad2/3 pathway down-regulates RUNX2 expression whereas the Smad1/5/8 pathway up-regulates and RUNX2 expression [16]. RUNX2 is essential for bone formation and a key controller of chondrocyte terminal differentiation [109] which can also be characterized by Col X expression. Because we observed high levels of Col X in TGF-β3-treated ADSCs, we suspect that TGF-β administration up-regulates RUNX2 expression through activation of the Smad1/5/8 pathway. The present study is extensive in terms of the number of genes monitored, each at 4 time points plus the day 0 baseline, which has provided an initial quantitative picture of the chondrogenic pattern elicited by the selected sets of CFs and their combination. However, we recommend considerable expansion of gene expression profiles for future studies to look for the exact molecular mechanisms underlying these effects by studying the expression pattern of not only Smad1/5/8 and RUNX2 as a function of time but also other intracellular factors shown to affect the TGF-β signaling pathway such as Smurf2 which blocks the Smad2/3 pathway [110].
Asc plays an important role in cartilage and bone formation [15]. In our study, Asc treatment up-regulated non-chondrogenic markers at every time point. Similarly, in vitro studies show that Asc induces ALP and Col X in chick chondrocytes [111,112]. Literature suggests that promotion of the terminally differentiated chondrocyte by Asc is mediated by the ECM, specifically collagens and proteoglycans, and involves interactions between cell surface receptors such as integrins and recognition peptides [113]. Interactions between integrins and ECM proteins prompt a signaling cascade that can activate RUNX2 [114]. As illustrated in Figure 10 these scenarios suggest that cell-matrix interactions and subsequent RUNX2 activation partially accounts for the effects of Asc on hADSC differentiation. Nonetheless, future studies should clarify the exact mode of action of Asc-induced hADSCs terminal differentiation by studying the impact of Asc on RUNX2 upregulation along with upregulation of chondrogenic mRNA indicators.
DT- and DTA-treated samples have positive indices at all-time points. This may be explained by the fact that Dex-mediated suppression of interleukin 1β and subsequent upregulation of Smad6 [115] to suppress RUNX2 expression counterbalances osteogenic impacts of TGF-β and Asc. Hence, where use of TGF-β or Asc is desirable the medium should be supplemented with Dex.
In addition to proper differentiation of SCs, the aim of ACTE is to develop functional tissues with appropriate mechanical properties. As assessed by AFM, none of our tissues developed elastic moduli in the 300 to 1000 kPa native AC range [116,117]. The respective 101 and 219 kPa values for TGF-β3 at day 3 and Asc at day 7 began to approach this range. As we reported previously [46], additional stimulation such as through application of cyclic hydrostatic pressure will be necessary to improve the elastic modulus to be on the scale of native AC. Our results show greater differences in elastic moduli earlier in culture, with all elastic moduli leveling out at 2 standard deviations greater than NC at day 25. In selecting appropriate CFs, chondrogenic expression must ultimately coincide with functional mechanical properties likely achieved with simultaneous mechanical stimulation.
We believe investigating each signaling molecule and its temporal effects on an extensive array of genetic markers and on tissue mechanical properties is necessary before complicating the investigation with CF combinations. For example, the exact role of Dex in modulating MSC chondrogenesis, its cross-talk with BMP-2 and TGF-β1, and the elasticity of the constructs obtained is still not fully understood [67]. Only when the roles of individual factors and their synergistic interplay are known as a function of time, using whole mRNA sequencing, can a gold standard differentiation cocktail be developed. Toward this aim, future studies are recommended using even larger sample sizes, and pooling from a greater number of micromass cultures, to assure that the large error bars observed in this type of data are because of sample heterogeneity and not the paucity in the total number of samples used in an analysis to create data to be represented.
Pretreatment prior to differentiation is an emerging research area in ACTE shown to increase cell regenerative potential of pretreated cells [118,119]. For example, pretreatment with 10-8 M Dex during the expansion phase downregulates genes correlated with apoptosis, upregulates genes correlated with proliferation and promotes chondrogenesis in hBMSCs [120]. We recommend studies to investigate hADSC pretreatment with individual and combined CFs using whole genome sequencing to clarify underlying molecular mechanisms controlling differentiation. The authors also acknowledge the need to go beyond quantitative analyses based only on genetic expression and determine actual protein expression and deposition. The AFM data provided represent an initial effort toward this end in that resultant mechanical properties of the ECM are closely tied to actual protein composition. To include the expression of every protein is beyond the scope of this study, but rather the focus is on a thorough temporal analysis of mRNA upregulation for a set of nine different markers, mechanical properties and the chondrogenic index. Investigators in future studies should include protein expression of chondrogenic and non-chondrogenic markers at the protein levels. This can be done by utilizing enzyme-linked immunosorbent assays (ELISAs) to measure ACAN , and VEGF [121], western blots to measure SOX9 [54], Col XI [122], lubricin [123] and osterix [124], a chloramine-T hydroxyproline assay to measure total collagen content, immunohistochemistry to measure collagen types I and X [21], and the QuantiChrom™ ALP Assay Kit to measure ALP. Of course any single one of these ancillary studies is an undertaking in itself and the authors recommend a series of careful studies to further clarify the overall chondrogenic picture.
Finally, temporal exposure to CFs is of interest [125]. In Buxton et al.’s study [126], collagen deposition was the same in hBMSCs supplemented with TGF-β1 for either the initial week of culture or for 3 weeks continuously and given no further improvement the results suggest that transition to another factor may improve other chondrogenic indices. Even though BMSCs and ADSCs are inherently different, we hypothesize that changing supplements throughout the culture period may best induce ADSC chondrogenesis since it mimics the native environment where certain signaling molecules are upregulated temporally [127]. By regulating introduction time points, we may provide the cells with factors they need temporally to maximize the expression of chondrogenic markers and minimize the expression of non-chondrogenic markers. Based on figure 9, we recommend DT be supplied for the first three days since it has the highest index at day 3. At day 3 to 7, despite a higher index for Dex compared to DTA we recommend DTA be supplied because Asc enhances Young’s moduli by 10-fold compared to Dex. From day 7 to the end of culture, use of Dex supplemented medium is advised because Dex has the highest indices at days 14 and 25. Based on our data, we calculated a hypothetical index that may be achieved by such a scenario. The hypothetical index is also shown in Figure 9 which may be calculated for any supplement switching scenario by taking an experimentally observed ratio of the latter to former day mRNA values for a second supplement in a series, and multiplying it by existing mRNA values at a given day for the first supplemented medium. For our hypothetical scenario, we would use the following set of equations:
This results in hypothetical 17, 20 and 13% increases in maximal calculated hypothetical indices for the temporal additions at days 7, 14 and 25, over that achieved by use of Dex alone. Our suggestion will need to be validated experimentally as we understand that these time-point ratios are not fully independent of earlier supplementation. At the same time, future studies should be directed to not only look into transient and continuous supplementation of other CFs, which have been shown to be efficient in inducing ADSC chondrogenesis such as BMP-6 [128], but also other chondrogenic such as Col II and RUNX2 and as well as other non-chondrogenic markers.
CONCLUSIONS
In this study, we compare the temporal impact of three CFs, Dex, TGF-β3, Asc and selected combinations, DT and DTA on ADSC mRNA expression of chondrogenic markers, tissue Young’s modulus, and mRNA expression of non-chondrogenic markers including hypertrophic, angiogenic, fibrogenic, and osteogenic markers. Based on our results, continuous supplementation of Dex, TGF-β3 and Asc induced the expression of both chondrogenic and non-chondrogenic markers. However, Dex induces significantly higher levels of chondrogenic markers such as ACAN and lubricin and results in the lowest expression of non-chondrogenic markers such as Col X, VEGF-A and ALP, whereas TGF-β3 has the opposite effect promoting hADSC expression of the most non-chondrogenic markers, specifically Col X and osterix. To a lower extent, Asc treatment also elicited the up-regulation of non-chondrogenic markers. As seen in DT- and DTA-treated samples, CFs studied here did not work in synergy to induce expression of the chondrogenic markers nor to suppress the expression of the non-chondrogenic markers. We suspect suppression of interleukin 1β and the up-regulation of RUNX2 through either the Smad pathway or cell-matrix interactions partially accounts for the effects of Dex, TGF-β3 and Asc on hADSC differentiation. Future work is recommended for an expanded set of markers to elucidate pathways and to employ the chondrogenic index as a means to assess optimal CF regimens including the shift from one set of CFs to another.
ACKNOWLEDGEMENTS
The authors acknowledge funding from NSF EAGER and GOALI grants CBET-1212573 and CBET-1606226, respectively, an NSF GRDS supplement CBET-1245188, the NIH Protein Biotechnology Training Program 24280305, a NASA Space Grant, a WSU DRADS fellowship, a Harold P. Curtis Scholarship for Chrystal Quisenberry, as well as salary support for Prof. Van Wie from USDA NIFA Hatch Project #WNP00807. We would also like to thank Regeneron Pharmaceuticals, Inc. for supplies and helpful discussions with Regeneron collaborators Dr. Vincent Idone and Hyon Kim. Finally, we would like to thank Haluk Beyenal, Cornelius Ivory, Eric Darling, Nicholas Labriola and Brandon Graham for their assistance in the protocol and/or assembly of the colloidal probes.
REFERENCES
- Longo UG, Petrillo S, Franceschetti E, Berton A, Maffulli N, et al. Stem Cells and Gene Therapy for Cartilage Repair. Stem Cells Int. 2012; 2012: 168385. Ref.: https://goo.gl/B8pCeK
- Buckwalter JA. Articular cartilage: injuries and potential for healing. J Orthop Sports Phys Ther. 1998; 28: 192-202. Ref.: https://goo.gl/QYV1E8
- Lee CR, Grodzinsky AJ, Hsu HP, Martin SD, Spector M. Effects of harvest and selected cartilage repair procedures on the physical and biochemical properties of articular cartilage in the canine knee. J Orthop Res. 2000; 18: 790-799. Ref.: https://goo.gl/poz8Kr
- Darling EM, Athanasiou KA. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J Orthop Res. 2005; 23: 425-432. Ref.: https://goo.gl/bztYZU
- Winter A, Breit S, Parsch D, Benz K, Steck E, et al. Cartilage-like gene expression in differentiated human stem cell spheroids: A comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum. 2003; 48: 418-429. Ref.: https://goo.gl/XMpcXN
- Grigolo B, Lisignoli G, Desando G, Cavallo C, Marconi E, et al. Osteoarthritis treated with mesenchymal stem cells on hyaluronan-based scaffold in rabbit. Tissue Eng Part C Methods. 2009; 15: 647-658. Ref.: https://goo.gl/CTeKm8
- Al Faqeh H, Nor Hamdan BM, Chen HC, Aminuddin BS, Ruszymah BH. The potential of intra-articular injection of chondrogenic-induced bone marrow stem cells to retard the progression of osteoarthritis in a sheep model. Exp Gerontol. 2012; 47: 458-464. Ref.: https://goo.gl/WDGPnX
- Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003; 48: 3464-3474. Ref.: https://goo.gl/6CnJo9
- Sato M, Uchida K, Nakajima H, Miyazaki T, Guerrero AR, et al. Direct transplantation of mesenchymal stem cells into the knee joints of Hartley strain guinea pigs with spontaneous osteoarthritis. Arthritis Res Ther. 2012; 14: 31. Ref.: https://goo.gl/mUC9vs
- Wakitani S, Nawata M, Tensho K, Okabe T, Machida H, et al. Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: three case reports involving nine defects in five knees. J Tissue Eng Regen Med. 2007; 1: 74-79. Ref.: https://goo.gl/YYmTif
- Lee RH, Kim B, Choi I, Kim H. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem. 2004; 14: 311-324. Ref.: https://goo.gl/pJY7WG
- Ogawa R, Mizuno S, Murphy GF, Orgill DP. The effect of hydrostatic pressure on three-dimensional chondroinduction of human adipose-derived stem cells. Tissue Eng Part A. 2009; 15: 2937-2945. Ref.: https://goo.gl/vu6nK1
- Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003; 5: 362-369. Ref.: https://goo.gl/oCTsHr
- Schmid TM, Linsenmayer TF. Immunohistochemical localization of short chain cartilage collagen (type X) in avian tissues. J Cell Biol. 1985; 100: 598-605. Ref.: https://goo.gl/6WtKcS
- Wegger I, Palludan B. Vitamin C deficiency causes hematological and skeletal abnormalities during fetal development in swine. J Nutr. 1994; 124: 241-248. Ref.: https://goo.gl/Q92ZLn
- van der Kraan PM, Davidson ENB, Blom A, van den Berg WB. TGF-beta signaling in chondrocyte terminal differentiation and osteoarthritis Modulation and integration of signaling pathways through receptor-Smads. Osteoarthritis Cartilage 2009; 17: 1539-1545. Ref.: https://goo.gl/yY2PDQ
- Walsh S, Jordan GR, Jefferiss C, Stewart K, Beresford JN. High concentrations of dexamethasone suppress the proliferation but not the differentiation or further maturation of human osteoblast precursors in vitro: relevance to glucocorticoid-induced osteoporosis. Rheumatology. 2001; 40: 74-83. Ref.: https://goo.gl/nhwSE6
- Hong L, Sultana H, Paulius K, Zhang G. Steroid regulation of proliferation and osteogenic differentiation of bone marrow stromal cells: a gender difference. J Steroid Biochem Mol Biol. 2009; 114: 180-185. Ref.: https://goo.gl/kiKjkH
- Derfoul A, Perkins GL, Hall DJ, Tuan RS. Glucocorticoids promote chondrogenic differentiation of adult human mesenchymal stem cells by enhancing expression of cartilage extracellular matrix genes. Stem Cells. 2006; 24: 1487-1495. Ref.: https://goo.gl/utBa5T
- Nazempour A, Wie BJ. Chondrocytes, Mesenchymal Stem Cells, and Their Combination in Articular Cartilage Regenerative Medicine. Ann Biomed Eng. 2016; 1325-1354. Ref.: https://goo.gl/tKMNpd
- Cals FL, Hellingman CA, Koevoet W, Baatenburg de Jong RJ, van Osch GJ. Effects of transforming growth factor-beta subtypes on in vitro cartilage production and mineralization of human bone marrow stromal-derived mesenchymal stem cells. J Tissue Eng Regen Med. 2012; 6: 68-76. Ref.: https://goo.gl/6Xqi3z
- Awad HA, Halvorsen YD, Gimble JM, Guilak F. Effects of transforming growth factor beta1 and dexamethasone on the growth and chondrogenic differentiation of adipose-derived stromal cells. Tissue Eng. 2003; 9: 1301-1312. Ref.: https://goo.gl/eXiXWo
- Altaf FM, Hering TM, Kazmi NH, Yoo JU, Johnstone B. Ascorbate-enhanced chondrogenesis of ATDC5 cells. Eur Cell Mater. 2006; 12: 64-70. Ref.: https://goo.gl/HzZ6tp
- Xu Y, Balooch G, Chiou M, Bekerman E, Ritchie RO, et al. Analysis of the material properties of early chondrogenic differentiated adipose-derived stromal cells (ASC) using an in vitro three-dimensional micromass culture system. Biochem Biophys Res Commun. 2007; 359: 311-316. Ref.: https://goo.gl/PAFcRF
- Stott NS, Jiang TX, Chuong CM. Successive formative stages of precartilaginous mesenchymal condensations in vitro: modulation of cell adhesion by Wnt-7A and BMP-2. J Cell Physiol. 1999; 180: 314-324. Ref.: https://goo.gl/mpFKzn
- Ahrens PB, Solursh M, Reiter RS. Stage-related capacity for limb chondrogenesis in cell culture. Dev Biol. 1977; 60: 69-82. Ref.: https://goo.gl/HScfBL
- Carlberg AL, Pucci B, Rallapalli R, Tuan RS, Hall DJ. Efficient chondrogenic differentiation of mesenchymal cells in micromass culture by retroviral gene transfer of BMP-2. Differentiation. 2001; 67: 128-138. Ref.: https://goo.gl/njGqNs
- Hou C, Zhang Z, Zhang Z, Wu P, Zhao X, et al. Presence and function of microRNA-92a in chondrogenic ATDC5 and adipose-derived mesenchymal stem cells. Mol Med Rep. 2015; 12: 4877-4886. Ref.: https://goo.gl/WV8bEL
- Yang JW, de Isla N, Huselstein C, Sarda-Kolopp MN, Li N, et al. Evaluation of human MSCs cell cycle, viability and differentiation in micromass culture. Biorheology. 2006; 43: 489-496. Ref.: https://goo.gl/JM39oi
- Zhang L, Su P, Xu C, Yang J, Yu W, et al. Chondrogenic differentiation of human mesenchymal stem cells: a comparison between micromass and pellet culture systems. Biotechnol Lett. 2010; 32: 1339-1346. Ref.: https://goo.gl/iqgdYb
- Johnston S, Siegel C. Comparison of a serum replacement (Omni Serum) and fetal bovine serum in cell cultures used to isolate herpes simplex virus from clinical specimens. J Clin Microbiol. 1990; 28: 643-645. Ref.: https://goo.gl/QrW1V5
- Vavken P, Arrich F, Pilz M, Dorotka R. An in vitro model of biomaterial-augmented microfracture including chondrocyte-progenitor cell interaction. Arch Orthop Trauma Surg. 2010; 130: 711-716. Ref.: https://goo.gl/zJDW5m
- Ryu JS, Jung YH, Cho MY, Yeo JE, Choi YJ, et al. Co-culture with human synovium-derived mesenchymal stem cells inhibits inflammatory activity and increases cell proliferation of sodium nitroprusside-stimulated chondrocytes. Biochem Biophys Res Commun. 2014; 447: 715-720. Ref.: https://goo.gl/3g751G
- Xu L, Wang Q, Xu F, Ye Z, Zhou Y, et al. Mesenchymal stem cells downregulate articular chondrocyte differentiation in noncontact coculture systems: implications in cartilage tissue regeneration. Stem Cells Dev. 2013; 22: 1657-1669. Ref.: https://goo.gl/bKYdTD
- Lai JH, Kajiyama G, Smith RL, Maloney W, Yang F. Stem cells catalyze cartilage formation by neonatal articular chondrocytes in 3D biomimetic hydrogels. Sci Rep. 2013; 3: 3553. Ref.: https://goo.gl/N4XY4D
- Lopa S, Colombini A, Sansone V, Preis FW, Moretti M. Influence on chondrogenesis of human osteoarthritic chondrocytes in co-culture with donor-matched mesenchymal stem cells from infrapatellar fat pad and subcutaneous adipose tissue. Int J Immunopathol Pharmacol. 2013; 26: 23-31. Ref.: https://goo.gl/BR8cs9
- Leyh M, Seitz A, Dürselen L, Schaumburger J, Ignatius A, et al. Subchondral bone influences chondrogenic differentiation and collagen production of human bone marrow-derived mesenchymal stem cells and articular chondrocytes. Arthritis Res Ther. 2014; 16: 453. Ref.: https://goo.gl/Fmy7TC
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008; 3: 1101-1108. Ref.: https://goo.gl/7K2R5h
- Harada M, Mitsuyama K, Yoshida H, Sakisaka S, Taniguchi E, et al. Vascular endothelial growth factor in patients with rheumatoid arthritis. Scand J Rheumatol. 1998; 27: 377-380. Ref.: https://goo.gl/RHxUFZ
- Abu-Lail NI, Camesano TA. The effect of solvent polarity on the molecular surface properties and adhesion of Escherichia coli. Colloids Surf B Biointerfaces. 2006; 51: 62-70. Ref.: https://goo.gl/KeDLSz
- Barquins M, Maugis D. Adhesive contact of axisymmetric punches on an elastic half-space-the modified Hertz-Hubers stress tensor for contacting spheres. Journal de Mecanique theorique et appliquee. 1982; 1: 331-357.
- Park B-J, Abu-Lail NI. Variations in the Nanomechanical Properties of Virulent and Avirulent Listeria monocytogenes. Soft matter. 2010; 6: 3898-3909. Ref.: https://goo.gl/eCJoX8
- Long R, Hall Matthew S, Wu M, Hui CY. Effects of Gel Thickness on Microscopic Indentation Measurements of Gel Modulus. Biophysical Journal. 2011; 101: 643-650. Ref.: https://goo.gl/PhzTet
- Santos J, Rebêlo L, Araujo A, Barrosa EB, de Sousa JS. Thickness-corrected model for nanoindentation of thin films with conical indenters. Soft Matter. 2012; 8: 4441-4448. Ref.: https://goo.gl/tNJVJN
- Fischer-Cripps AC. The Hertzian contact surface. Journal of Materials Science. 1999; 34: 129-137. Ref.: https://goo.gl/MRyHZt
- Nazempour A, Quisenberry CR, Van Wie BJ, Abu-Lail NI. Nanomechanics of engineered articular cartilage: synergistic influences of transforming growth factor-β3 and oscillating pressure. J Nanosci Nanotechnol. 2016; 16: 3136-3145. Ref.: https://goo.gl/tKpzxd
- Yuan JS, Reed A, Chen F, Stewart CN Jr. Statistical analysis of real-time PCR data. BMC Bioinformatics. 2006; 7: 85. Ref.: https://goo.gl/mJ47oj
- Kern JA, Lamb RJ, Reed JC, Daniele RP, Nowell PC. Dexamethasone inhibition of interleukin 1 beta production by human monocytes. Posttranscriptional mechanisms. J Clin Invest. 1998; 81: 237-244. Ref.: https://goo.gl/Ph3eKD
- Stewart AA, Byron CR, Pondenis HC, Stewart MC. Effect of dexamethasone supplementation on chondrogenesis of equine mesenchymal stem cells. Am J Vet Res. 2008; 69: 1013-1021. Ref.: https://goo.gl/Ltk1H1
- Hall BK. Chapter 23-Cartilage Diversity. Bones and Cartilage (Second Edition). San Diego: Academic Press. 2015; 387-400.
- Acharya C, Adesida A, Zajac P, Mumme M, Riesle J, et al. Enhanced chondrocyte proliferation and mesenchymal stromal cells chondrogenesis in coculture pellets mediate improved cartilage formation. J Cell Physiol. 2012; 227: 88-97. Ref.: https://goo.gl/XWE97D
- Bruce SJ, Butterfield NC, Metzis V, Town L, McGlinn E, et al. Inactivation of Patched1 in the mouse limb has novel inhibitory effects on the chondrogenic program. J Biol Chem. 2010; 285: 27967-27981. Ref.: https://goo.gl/9KRgv9
- Kalwitz G, Neumann K, Ringe J, Sezer O, Sittinger M, et al. Chondrogenic differentiation of human mesenchymal stem cells in micro-masses is impaired by high doses of the chemokine CXCL7. Journal of Tissue Engineering and Regenerative Medicine. 2011; 5: 50-59. Ref.: https://goo.gl/R1ox1N
- Cooke ME, Allon AA, Cheng T, Kuo AC, Kim HT, et al. Structured three-dimensional co-culture of mesenchymal stem cells with chondrocytes promotes chondrogenic differentiation without hypertrophy. Osteoarthritis Cartilage. 2011; 19: 1210-1218. Ref.: https://goo.gl/Ehp8qj
- Tanaka H, Murphy CL, Murphy C, Kimura M, Kawai S, et al. Chondrogenic differentiation of murine embryonic stem cells: effects of culture conditions and dexamethasone. J Cell Biochem. 2004; 93: 454-462. Ref.: https://goo.gl/3FK6sP
- Zhou H, Mak W, Kalak R, Street J, Fong-Yee C, et al. Glucocorticoid-dependent Wnt signaling by mature osteoblasts is a key regulator of cranial skeletal development in mice. Development. 2009; 136: 427-436. Ref.: https://goo.gl/8Xw9Ty
- Lee MJ, Fried SK. The glucocorticoid receptor, not the mineralocorticoid receptor, plays the dominant role in adipogenesis and adipokine production in human adipocytes. Int J Obes (Lond). 2014; 38: 1228-1233. Ref.: https://goo.gl/gTL21q
- Huang JI, Zuk PA, Jones NF, Zhu M, Lorenz HP, et al. Chondrogenic potential of multipotential cells from human adipose tissue. Plast Reconstr Surg. 2004; 113: 585-594. Ref.: https://goo.gl/q6ZTy4
- Kim HJ, Im GI. Chondrogenic differentiation of adipose tissue-derived mesenchymal stem cells: greater doses of growth factor are necessary. J Orthop Res. 2009; 27: 612-619. Ref.: https://goo.gl/2grnMs
- Akiyama H. Control of chondrogenesis by the transcription factor Sox9. Mod Rheumatol. 2008; 18: 213-219. Ref.: https://goo.gl/1Zidcn
- Diao HJ, Yeung CW, Yan CH, Chan GC, Chan BP. Bidirectional and mutually beneficial interactions between human mesenchymal stem cells and osteoarthritic chondrocytes in micromass co-cultures. Regen Med. 2013; 8: 257-269. Ref.: https://goo.gl/x3ryDx
- von der Mark K, Gauss V, von der Mark H, Müller P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature. 1977; 267: 531-532. Ref.: https://goo.gl/dgXyQ1
- Hao H, Chen G, Liu J, Ti D, Zhao Y, et al. Culturing on Wharton's jelly extract delays mesenchymal stem cell senescence through p53 and p16INK4a/pRb pathways. Plos One. 2013; 8: 13. Ref.: https://goo.gl/1VQYzp
- Kawakami Y, Rodriguez-Leon J, Izpisua Belmonte JC. The role of TGFbetas and Sox9 during limb chondrogenesis. Curr Opin Cell Biol. 2006; 18: 723-729. Ref.: https://goo.gl/KeXmDG
- Bell DM, Leung KK, Wheatley SC, Ng LJ, Zhou S, et al. SOX9 directly regulates the type-II collagen gene. Nat Genet. 1997; 16: 174-178. Ref.: https://goo.gl/Wg7v9R
- Rich JT, Rosova I, Nolta JA, Myckatyn TM, Sandell LJ, et al. Upregulation of Runx2 and Osterix during in vitro chondrogenesis of human adipose-derived stromal cells. Biochem Biophys Res Commun. 2008; 372: 230-235. Ref.: https://goo.gl/r6rpbM
- Shintani N, Hunziker EB. Differential effects of dexamethasone on the chondrogenesis of mesenchymal stromal cells: influence of microenvironment, tissue origin and growth factor. Eur Cell Mater. 2011; 22: 302-319. Ref.: https://goo.gl/gM4uyi
- Diekman BO, Rowland CR, Lennon DP, Caplan AI, Guilak F. Chondrogenesis of Adult Stem Cells from Adipose Tissue and Bone Marrow: Induction by Growth Factors and Cartilage-Derived Matrix. Tissue Engineering Part A. 2010; 16: 523-533. Ref.: https://goo.gl/V7vvwk
- Aung A, Gupta G, Majid G, Varghese S. Osteoarthritic chondrocyte-secreted morphogens induce chondrogenic differentiation of human mesenchymal stem cells. Arthritis Rheum. 2011; 63: 148-158. Ref.: https://goo.gl/8QsMKd
- Jay GD, Waller KA. The biology of lubricin: near frictionless joint motion. Matrix Biol. 2014; 39: 17-24. Ref.: https://goo.gl/RHZHnD
- Waller KA, Zhang LX, Elsaid KA, Fleming BC, Warman ML, et al. Role of lubricin and boundary lubrication in the prevention of chondrocyte apoptosis. Proceedings of the National Academy of Sciences. 2013; 110: 5852-5857. Ref.: https://goo.gl/Va5tbac
- Jones AR, Flannery CR. Bioregulation of lubricin expression by growth factors and cytokines. Eur Cell Mater. 2007; 13: 40-45. Ref.: https://goo.gl/nE4N29
- Eyre D. Articular cartilage and changes in Arthritis: Collagen of articular cartilage. Arthritis Research & Therapy. 2001; 4: 1-6. Ref.: https://goo.gl/k7dQCf
- Sophia Fox AJ, Bedi A, Rodeo SA. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health. 2009; 1: 461-468. Ref.: https://goo.gl/dL8txZ
- Spranger J. The type XI collagenopathies. Pediatr Radiol. 1998; 28: 745-750. Ref.: https://goo.gl/J45AZN
- Kwan AP, Cummings CE, Chapman JA, Grant ME. Macromolecular organization of chicken type X collagen in vitro. J Cell Biol. 1991; 114: 597-604. Ref.: https://goo.gl/zgy2wx
- Mwale F, Stachura D, Roughley P, Antoniou J. Limitations of using aggrecan and type X collagen as markers of chondrogenesis in mesenchymal stem cell differentiation. J Orthop Res. 2006; 24: 1791-1798. Ref.: https://goo.gl/i3jy5n
- Bengoetxea H, Argandoña EG, Lafuente Jé V. Effects of Visual Experience on Vascular Endothelial Growth Factor Expression during the Postnatal Development of the Rat Visual Cortex. Cereb Cortex. 2008; 18: 1630-1639. Ref.: https://goo.gl/HyoGKP
- Giatromanolaki A, Sivridis E, Athanassou N, Zois E, Thorpe PE, et al. The angiogenic pathway ‘vascular endothelial growth factor/flk-1(KDR)-receptor’ in rheumatoid arthritis and osteoarthritis. The Journal of Pathology. 2001; 194: 101-108. Ref.: https://goo.gl/CJLPpt
- Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004; 109: 1292-1298. Ref.: https://goo.gl/1NioEG
- Lee CS, Watkins E, Burnsed OA, Schwartz Z, Boyan BD. Tailoring adipose stem cell trophic factor production with differentiation medium components to regenerate chondral defects. Tissue Eng Part A. 2013; 19: 1451-1464. Ref.: https://goo.gl/t1cELW
- Sandell LJ, Aigner T. Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis. Arthritis Res. 2001; 3: 107-113. Ref.: https://goo.gl/rfBqgB
- Diekman BO, Estes BT, Guilak F. The effects of BMP6 overexpression on adipose stem cell chondrogenesis: Interactions with dexamethasone and exogenous growth factors. J Biomed Mater Res A. 2010; 93: 994-1003. Ref.: https://goo.gl/k9ujzV
- Tapp H, Deepe R, Ingram JA, Kuremsky M, Hanley EN Jr, et al. Adipose-derived mesenchymal stem cells from the sand rat: transforming growth factor beta and 3D co-culture with human disc cells stimulate proteoglycan and collagen type I rich extracellular matrix. Arthritis Res Ther. 2008; 10: 89. Ref.: https://goo.gl/cc9aPq
- Birmingham E, Niebur GL, McHugh PE, Shaw G, Barry FP, et al. Osteogenic differentiation of mesenchymal stem cells is regulated by osteocyte and osteoblast cells in a simplified bone niche. Eur Cell Mater. 2012; 23: 13-27. Ref.: https://goo.gl/iTasgi
- Miao D, Scutt A. Histochemical localization of alkaline phosphatase activity in decalcified bone and cartilage. J Histochem Cytochem. 2002; 50: 333-340. Ref.: https://goo.gl/9hZLFH
- Cimmino MA, Buffrini L, Barisone G, Bruzzone M, Accardo S. Alkaline phosphatase activity in the serum of patients with rheumatoid arthritis. Z Rheumatol. 1990; 49: 143-146. Ref.: https://goo.gl/EPFDBA
- Langenbach F, Handschel J. Effects of dexamethasone, ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Research & Therapy. 2013; 4: 117-117. Ref.: https://goo.gl/7avFxi
- Westhrin M, Xie M, Olderoy MO, Sikorski P, Strand BL, et al. Osteogenic differentiation of human mesenchymal stem cells in mineralized alginate matrices. PLoS One. 2015; 10: 0120374. Ref.: https://goo.gl/KGcCQG
- Cruz AC, Silva ML, Caon T, Simões CM. Addition of bone morphogenetic protein type 2 to ascorbate and beta-glycerophosphate supplementation did not enhance osteogenic differentiation of human adipose-derived stem cells. J Appl Oral Sci. 2012; 20: 628-635. Ref.: https://goo.gl/u1itzK
- Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002; 108: 17-29. Ref.: https://goo.gl/YMfdA2
- Zhu F, Friedman MS, Luo W, Woolf P, Hankenson KD. The transcription factor osterix (SP7) regulates BMP6-induced human osteoblast differentiation. J Cell Physiol. 2012; 227: 2677-2685. Ref.: https://goo.gl/tW4sYJ
- Nishimura R, Wakabayashi M, Hata K, Matsubara T, Honma S, et al. Osterix regulates calcification and degradation of chondrogenic matrices through matrix metalloproteinase 13 (MMP13) expression in association with transcription factor Runx2 during endochondral ossification. J Biol Chem. 2012; 287: 33179-33190. Ref.: https://goo.gl/Pwe3xi
- Aguiari P, Leo S, Zavan B, Vindigni V, Rimessi A, et al. High glucose induces adipogenic differentiation of muscle-derived stem cells. Proc Natl Acad Sci U S A. 2008; 105: 1226-1231. Ref.: https://goo.gl/PwnAk8
- Chuang CC, Yang RS, Tsai KS, Ho FM, Liu SH. Hyperglycemia enhances adipogenic induction of lipid accumulation: involvement of extracellular signal-regulated protein kinase 1/2, phosphoinositide 3-kinase/Akt, and peroxisome proliferator-activated receptor gamma signaling. Endocrinology. 2007; 148: 4267-4275. Ref.: https://goo.gl/TMWZnD
- Tsai TL, Manner PA, Li WJ. Regulation of mesenchymal stem cell chondrogenesis by glucose through protein kinase C/transforming growth factor signaling. Osteoarthritis Cartilage. 2013; 21: 368-376. Ref.: https://goo.gl/RsN5u9
- Advani S, LaFrancis D, Bogdanovic E, Taxel P, Raisz LG, et al. Dexamethasone suppresses in vivo levels of bone collagen synthesis in neonatal mice. Bone. 1997; 20: 41-46. Ref.: https://goo.gl/yz3ij7
- Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, et al. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Engineering. 1998; 4: 415-428. Ref.: https://goo.gl/FZFNR1
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284: 143-147. Ref.: https://goo.gl/xQKrQG
- Towle CA, Hung HH, Bonassar LJ, Treadwell BV, Mangham DC. Detection of interleukin-1 in the cartilage of patients with osteoarthritis: a possible autocrine/paracrine role in pathogenesis. Osteoarthritis and Cartilage. 1997; 5: 293-300. Ref.: https://goo.gl/FraFzS
- Huh JE, Koh PS, Seo BK, Park YC, Baek YH, et al. Mangiferin reduces the inhibition of chondrogenic differentiation by IL-1beta in mesenchymal stem cells from subchondral bone and targets multiple aspects of the Smad and SOX9 pathways. Int J Mol Sci. 2014; 15: 16025-16042. Ref.: https://goo.gl/bG1sBe
- Kunz D, Walker G, Eberhardt W, Pfeilschifter J. Molecular mechanisms of dexamethasone inhibition of nitric oxide synthase expression in interleukin 1 beta-stimulated mesangial cells: evidence for the involvement of transcriptional and posttranscriptional regulation. Proceedings of the National Academy of Sciences. 1996; 93: 255-259. Ref.: https://goo.gl/xr1qbL
- Tangtrongsup S, Kisiday JD. Effects of Dexamethasone Concentration and Timing of Exposure on Chondrogenesis of Equine Bone Marrow-Derived Mesenchymal Stem Cells. Cartilage. 2016; 7: 92-103. Ref.: https://goo.gl/ScQCAh
- Lee SW, Tsou AP, Chan H, Thomas J, Petrie K, et al. Glucocorticoids selectively inhibit the transcription of the interleukin 1 beta gene and decrease the stability of interleukin 1 beta mRNA. Proc Natl Acad Sci U S A. 1988; 85: 1204-1208. Ref.: https://goo.gl/q4FRna
- Leonard CM, Fuld HM, Frenz DA, Downie SA, Massagué J, et al. Role of transforming growth factor-beta in chondrogenic pattern formation in the embryonic limb: stimulation of mesenchymal condensation and fibronectin gene expression by exogenenous TGF-beta and evidence for endogenous TGF-beta-like activity. Dev Biol. 1991; 145: 99-109. Ref.: https://goo.gl/DiU7mqb
- Song JJ, Aswad R, Kanaan RA, Rico MC, Owen TA, et al. Connective tissue growth factor (CTGF) acts as a downstream mediator of TGF-beta1 to induce mesenchymal cell condensation. J Cell Physiol. 2007; 210: 398-410. Ref.: https://goo.gl/GRRnTH
- Hinck AP. Structural studies of the TGF-betas and their receptors - insights into evolution of the TGF-beta superfamily. FEBS Lett. 2012; 586: 1860-1870. Ref.: https://goo.gl/3xdcFi
- ten Dijke P, Yamashita H, Ichijo H, Franzén P, Laiho M, et al. Characterization of type I receptors for transforming growth factor-beta and activin. Science. 1994; 264: 101-104. Ref.: https://goo.gl/2iEMGc
- Hecht J, Seitz V, Urban M, Wagner F, Robinson PN, et al. Detection of novel skeletogenesis target genes by comprehensive analysis of a Runx2(-/-) mouse model. Gene Expr Patterns. 2007; 7: 102-112. Ref.: https://goo.gl/hiWmYh
- Wu Q, Kim KO, Sampson ER, Chen D, Awad H, et al. Induction of an osteoarthritis-like phenotype and degradation of phosphorylated Smad3 by Smurf2 in transgenic mice. Arthritis Rheum. 2008; 58: 3132-3144. Ref.: https://goo.gl/7SH4Ns
- Habuchi H, Conrad HE, Glaser JH. Coordinate regulation of collagen and alkaline phosphatase levels in chick embryo chondrocytes. J Biol Chem. 1985; 260: 13029-13034. Ref.: https://goo.gl/NzxwKB
- Leboy PS, Vaias L, Uschmann B, Golub E, Adams SL, et al. Ascorbic acid induces alkaline phosphatase, type X collagen, and calcium deposition in cultured chick chondrocytes. J Biol Chem. 1989; 264: 17281-17286. Ref.: https://goo.gl/MKrR3F
- Farquharson C, Berry JL, Mawer EB, Seawright E, Whitehead CC. Ascorbic acid-induced chondrocyte terminal differentiation: the role of the extracellular matrix and 1,25-dihydroxyvitamin D. Eur J Cell Biol. 1998; 76: 110-118. Ref.: https://goo.gl/xHRN6D
- Li Y, Ge C, Franceschi RT. Differentiation-dependent association of phosphorylated extracellular signal-regulated kinase with the chromatin of osteoblast-related genes. J Bone Miner Res. 2010; 25: 154-163. Ref.: https://goo.gl/472SbC
- Kaiser M, Haag J, Soder S, Bau B, Aigner T. Bone morphogenetic protein and transforming growth factor beta inhibitory Smads 6 and 7 are expressed in human adult normal and osteoarthritic cartilage in vivo and are differentially regulated in vitro by interleukin-1beta. Arthritis Rheum. 2004; 50: 3535-3540. Ref.: https://goo.gl/79L8T5b
- Mansour J. Biomechanics of Cartilage. In: Oatis CA, editor. Kinesiology: the Mechanics and Pathomechanics of Human Movement. Philadelphia: Lippincott Williams and Wilkins. 2003; 66-79. Ref.: https://goo.gl/6E2U2N
- Ateshian G, Hung C. Functional properties of native articular cartilage. Functional Tissue Engineering. 2003; 46-68. Ref.: https://goo.gl/u3Vzhc
- Cui JH, Park SR, Park K, Choi BH, Min BH. Preconditioning of mesenchymal stem cells with low-intensity ultrasound for cartilage formation in vivo. Tissue Eng. 2007; 13: 351-360. Ref.: https://goo.gl/6ZejUD
- Lysdahl H, Baatrup A, Foldager CB, Bünger C. Preconditioning human mesenchymal stem cells with a low concentration of BMP2 stimulates proliferation and osteogenic differentiation in vitro. Biores Open Access. 2014; 3: 278-285. Ref.: https://goo.gl/5isdsY
- Xiao Y, Peperzak V, van Rijn L, Borst J, de Bruijn JD. Dexamethasone treatment during the expansion phase maintains stemness of bone marrow mesenchymal stem cells. J Tissue Eng Regen Med. 2010; 4: 374-386. Ref.: https://goo.gl/YDwchm
- Lee SS, Joo YS, Kim WU, Min DJ, Min JK, et al. Vascular endothelial growth factor levels in the serum and synovial fluid of patients with rheumatoid arthritis. Clin Exp Rheumatol. 2001; 19: 321-324. Ref.: https://goo.gl/BfE6ZC
- Shanmugasundaram S, Logan-Mauney S, Burgos K, Nurminskaya M. Tissue transglutaminase regulates chondrogenesis in mesenchymal stem cells on collagen type XI matrices. Amino Acids. 2012; 42: 1045-1053. Ref.: https://goo.gl/S8EfNc
- Ai M, Cui Y, Sy MS, Lee DM, Zhang LX, et al. Anti-lubricin monoclonal antibodies created using lubricin-knockout mice immunodetect lubricin in several species and in patients with healthy and diseased joints. PLoS One. 2015; 10: 0116237. Ref.: https://goo.gl/Bpy9Lo
- Han Y, Kim CY, Cheong H, Lee KY. Osterix represses adipogenesis by negatively regulating PPARgamma transcriptional activity. Sci Rep. 2016; 6: 35655. Ref.: https://goo.gl/ZCjPkk
- Yang Z, Zou Y, Guo XM, Tan HS, Denslin V, et al. Temporal activation of beta-catenin signaling in the chondrogenic process of mesenchymal stem cells affects the phenotype of the cartilage generated. Stem Cells Dev. 2012; 21: 1966-1976. Ref.: https://goo.gl/tdD53N
- Buxton AN, Bahney CS, Yoo JU, et al. Temporal exposure to chondrogenic factors modulates human mesenchymal stem cell chondrogenesis in hydrogels. Tissue Eng Part A. 2011; 17: 371-380. Ref.: https://goo.gl/u6DroQ
- Chimal-Monroy J, Rodriguez-Leon J, Montero JA, Gañan Y, Macias D, et al. Analysis of the molecular cascade responsible for mesodermal limb chondrogenesis: Sox genes and BMP signaling. Dev Biol. 2003; 257: 292-301. Ref.: https://goo.gl/jLfk2a
- Estes BT, Wu AW, Guilak F. Potent induction of chondrocytic differentiation of human adipose-derived adult stem cells by bone morphogenetic protein 6. Arthritis Rheum. 2006; 54: 1222-1232. Ref.: https://goo.gl/wrAoiR