Abstract
Review Article
Surgical Fetal Stem Cell Transplant into Heart Failure Patients Long-term Results at 14 Years
Federico Benetti*, Luis Geffner, Yan Duarte and Ernesto Peñaherrera
Published: 08 January, 2025 | Volume 9 - Issue 1 | Pages: 001-005
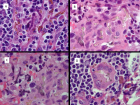
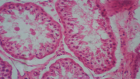
Direct myocardial transplant of HFDSCs (human fetal derived stem cells) by open chest surgical procedure was performed in 10 patients with Heart Failure (HF) due to no ischemic, no chagasic dilated cardiomyopathy. All 10 patients survived the operation. At 40 months, the mean (±SD) NYHA class decreased from 3.4 ± 0.5 to 1.33 ± 0.5 (p = .001); the mean EF increased 31%, from 26.6% ± F) 34.8% ± 7.2% (p = .005); and the mean ETT increased 291.3%, from 4.25 minutes to 16.63 minutes (128.9% increase in metabolic equivalents, from 2.46 to 5.63) (p < .0001); the mean LVEDD decreased 15%, from 6.85 ± 0.6 cm to 5.80 ± 0.58 cm (p < .001); mean performance in the 6-minute walk test increased by 43.2%, from 251 ± 113.1 seconds to 360 0 seconds (p = .01); the mean distance increased 64.4%, from 284.4 144.9 m to 468.2 ± 89.8 m (p = .004); and the mean result in the Minnesota test decreased from 71 ± 27.3 to 6 ± 5.9
(p < .001). Six patients survived after 40 months; 5 of them had complete reverse remodeling after 3 months after transplants. The average age at the moment of the transplants was 62 years (s/d 11.6).
Results: The first patient died at 5,4 years for an infection; the second patient died at,7,4 years for heart failure; the third patient died at 8,4 years for heart failure; the fourth patient died at 10 years for heart failure and the fifth patient died at 14,4 years after transplant at the age of 83 for heart failure. The average age at the moment of death was 70 years (s/d12.9). The survival rate at 4 years was 100% (K/M) and at 14 years (25%K/M).
Conclusion: These initial worldwide experiences with the surgical direct transplant of liver fetal stem cells in patients with end-stage HF shows clearly the positive effect in the reverse remodeling of the left ventricle of 50% of the cohort and excellent long-term results in these types of patients opening a new avenue for treating end-stage HF patients without any other option of treatment.
Read Full Article HTML DOI: 10.29328/journal.jsctt.1001045 Cite this Article Read Full Article PDF
Keywords:
Heart failure stem cells; Idiopathic cardiomyopathy and stem cells; Surgical stem cells in heart failure patients; Fetal stem cells in heart failure patients; Long-term follow-up of surgical fetal stem cell transplants; Fetal cells and heart failure term results
References
- Remme WJ, Swedberg K, Task force for the diagnosis and treatment of chronic heart failure, European Society of Cardiology. Guidelines for the diagnosis and treatment of chronic heart failure. Eur Heart J. 2001;22:1527-1560. Available from: https://doi.org/10.1053/euhj.2001.2783
- Colucci W, Braunwald E. Pathophysiology of heart failure. In: Braunwald E, editor. Heart Disease. 5th ed. Philadelphia: WB Saunders; 1997;360-393.
- Carbajal EV, Deedwani PC. Congestive heart failure. In: Deedwani PC, editor. Current Diagnosis and Treatment in Cardiology. 2nd ed. New York: McGraw-Hill; 2003;18.
- Bolling SF, Pagani FD, Deeb GM. Intermediate-term outcome of mitral reconstruction in cardiomyopathy. J Thorac Cardiovasc Surg. 1998;115(2):381-386. Available from: https://doi.org/10.1016/s0022-5223(98)70282-x
- Wang JS, Shum D, Galipeau J. Marrow stromal cells for cellular cardiomyoplasty: feasibility and potential clinical advantages. J Thorac Cardiovasc Surg. 2000;120(5):999-1005. Available from: https://doi.org/10.1067/mtc.2000.110250
- Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9(6):702-712. Available from: https://doi.org/10.1038/nm0603-702
- Orlic D, Kassutra J, Chimenti S, Jakoniuk I, Anderson SM, Li B. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701-705. Available from: https://doi.org/10.1038/35070587
- Edelberg JM, Tang L, Hattori K, Lyden D, Rafii S. Young adult bone marrow-derived endothelial precursor cells restore aging-impaired cardiac angiogenic function. Circ Res. 2002;E89-E93. Available from: https://doi.org/10.1161/01.RES.0000020861.20064.7E
- Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Isliura A, et al. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362-367. Available from: https://doi.org/10.1182/blood.V92.2.362
- Sata M, Saiura A, Kunisato A, Tojo A, Okara S, Tokuhisa T, et al. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med. 2002;8:403-409. Available from: https://doi.org/10.1038/nm0402-403
- Otani A, Kinder K, Ewalt K, Otero S, Tokuhisa T, Tirai H, et al. Bone marrow-derived stem cells target retinal astrocytes and can promote or inhibit retinal angiogenesis. Nat Med. 2002;8:1004-1010. Available from: https://doi.org/10.1038/nm744
- Young PP, Hofling AA, Sands MS. VEGF increases engraftment of bone marrow-derived endothelial progenitor cells (EPCs) into vasculature of newborn murine recipients. Proc Natl Acad Sci U S A. 2002;99:11951-11956. Available from: https://doi.org/10.1073/pnas.182215799
- Nigro P, Bassetti B, Cavallotti L, Catto V, Carbucicchio C, Pompilio G. Cell therapy for heart disease after 15 years: Unmet expectations. Pharmacol Res. 2018 Jan;127:77-91. Available from: https://doi.org/10.1016/j.phrs.2017.02.015
- Yagyu T, Yasuda S, Nagaya N, Doi K, Nakatani T, Satomi K, et al. Long-term results of intracardiac mesenchymal stem cell transplantation in patients with cardiomyopathy. Circ J. 2019 Jun 25;83(7):1590-1599. Available from: https://doi.org/10.1253/circj.CJ-18-1179
- Nakamura K, Murry CE. Function follows form - A review of cardiac cell therapy. Circ J. 2019 Nov 25;83(12):2399-2412. Available from: https://doi.org/10.1253/circj.cj-19-0567
- Bartunek J, Terzic A, Davison BA, Behfar A, Sanz-Ruiz R, Wojakowski W, et al. Cardiopoietic stem cell therapy in ischemic heart failure: long-term clinical outcomes. ESC Heart Fail. 2020;7(6):3345-3354. Available from: https://doi.org/10.1002/ehf2.13031
- Le DC, Bui HT, Vu YT, Vo QD. Induced pluripotent stem cell therapies in heart failure treatment: A meta-analysis and systematic review. Regen Med. 2024;19(9-10):1-13. Available from: https://doi.org/10.1080/17460751.2024.2393558
- Benetti F, Peñherrera E, Maldonado T, Vera YD, Subramanian V, Geffner L. Direct myocardial implantation of human fetal stem cells in heart failure patients: long-term results. Heart Surg Forum. 2010 Feb;13(1):E31-5. Available from: https://doi.org/10.1532/hsf98.20091130
- Meerson FZ. The myocardium in hyperfunction, hypertrophy, and heart failure. Circ Res. 1969;25(suppl 2):1-163. Available from: https://pubmed.ncbi.nlm.nih.gov/4240407/
- Zak R. Cardiac hypertrophy: biochemical and cellular relationships. Hosp Pract. 1983;18:85-97. Available from: https://doi.org/10.1080/21548331.1983.11702494
- Colucci WS, Braunwald E. Pathophysiology of heart failure. In: Braunwald E, editor. Braunwald’s Heart Disease. 5th ed. Saunders; 1997.
- Wynne J, Braunwald E. The cardiomyopathies and myocarditis. In: Braunwald E, editor. Braunwald’s Heart Disease. 5th ed. Saunders; 1997.
- Prokop D. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71-74. Available from: https://doi.org/10.1126/science.276.5309.71
- Boxall SA, Jones E. Markers for characterization of bone marrow multipotential stromal cells. Stem Cells Int. 2012;2012:975871. Available from: https://doi.org/10.1155/2012/975871
- Masson NM, Currie IS, Terrace JD, Garden OJ, Parks RW, Ross JA. Hepatic progenitor cells in human fetal liver express the oval cell marker Thy-1. Am J Physiol Gastrointest Liver Physiol. 2006 Jul;291(1):G45-54. Available from: https://doi.org/10.1152/ajpgi.00465.2005
- Pittenger MF, Makay A, Beck SC, Jaiwasal RK, Douglas R, Mosca JD, et alDR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. Available from: https://doi.org/10.1126/science.284.5411.143
- Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;105(5):697-705. Available from: https://doi.org/10.1172/jci5298
- Xu M, Wani M, Dai YS, Wang J, Yan M, Ayub A, et al. Differentiation of bone marrow stromal cells into cardiac phenotype requires intracellular communication with myocytes. Circulation. 2004;110:2658-2665. Available from: https://doi.org/10.1161/01.cir.0000145609.20435.36
- Chedway EG, Wang JS, Nguyen DM, Shum-Tim J, Chiu RCJ. Incorporation and integration of implanted myogenic and stem cells into native myocardial fibers: anatomic basis for functional improvements. J Thorac Cardiovasc Surg. 2002;124(3):584-590. Available from: https://doi.org/10.1067/mtc.2002.122544
- Pittenger MF, Bradley JM. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9-20. Available from: https://doi.org/10.1161/01.RES.0000135902.99383.6f
- Yau TM, Tomita S, Weisel RD, Jia ZQ, Tumiati LC, Mickle DA, et al. Beneficial effect of autologous cell transplantation on infarcted heart function: comparison between bone marrow stromal cells and heart cells. Ann Thorac Surg. 2003;75:169-177. Available from: https://doi.org/10.1016/s0003-4975(02)04290-x
- Fazel S, Tang GHL, Angoulvant D. Current status of cellular therapy for ischemic heart disease. Ann Thorac Surg. 2005 Jun;79(6):S2238-47. Available from: https://doi.org/10.1016/j.athoracsur.2005.02.085
- Sakakibara Y, Nishimura K, Tambara K, Yamamoto M, Lu F, Tabata Y, et al. Prevascularization with gelatin microspheres containing basic fibroblast growth factor enhances the benefits of cardiomyocyte transplantation. J Thorac Cardiovasc Surg. 2002;124(1):50-56. Available from: https://doi.org/10.1067/mtc.2002.121293
- Yamamoto M, Sakakibara Y, Nishimura K, Komeda M, Tabata Y. Improved therapeutic efficacy in cardiomyocyte transplantation for myocardial infarction with release system of basic fibroblast growth factor. Artif Organs. 2003;27(2):181-184. Available from: https://doi.org/10.1046/j.1525-1594.2003.06993.x
- Tambara K, Sakakibara Y, Sakaguchi G, Lu F, Premaratne GU, Lin X, et al. Transplanted skeletal myoblasts can fully replace the infarcted myocardium when they survive in the host in large numbers. Circulation. 2003;108(Suppl II):II259-II263. Available from: https://doi.org/10.1161/01.cir.0000087430.17543.b8
- Nakajima H, Sakakibara Y, Tambara K, Iwakura A, Doi K, Marui A, et al. Therapeutic angiogenesis by the controlled release of basic fibroblast growth factor for ischemic limb and heart injury: toward safety and minimal invasiveness. J Artif Organs. 2004;58-61. Available from: https://doi.org/10.1007/s10047-004-0252-1
- Marui A, Tabata Y, Kojima S, Yamamoto M, Tambara K, Nishina T, et al. A novel approach to therapeutic angiogenesis for patients with critical limb ischemia by sustained release of basic fibroblast growth factor using biodegradable gelatin hydrogel: an initial report of the phase I-IIa study. Circ J. 2007;71(8):1181-6. Available from: https://doi.org/10.1253/circj.71.1181
- Komeda M, Marui A, Tambara K, Yamamoto M, Saji Y, Nishina T, et al. Biologic anastomosis: the first case of biologic coronary bypass surgery. J Thorac Cardiovasc Surg. 2009;138:775-777. Available from: https://doi.org/10.1016/j.jtcvs.2008.05.066
- Sakakibara Y, Tambara K, Sakaguchi G, Lu F, Yamamoto M, Nishimura K, et al. Toward surgical angiogenesis using slow-released basic fibroblast growth factor. Eur J Cardiothorac Surg. 2003;24(1):105-112. Available from: https://doi.org/10.1016/s1010-7940(03)00159-3
- Tambara K, Tabata Y, Komeda M. Factors related to the efficacy of skeletal muscle cell transplantation and future approaches with controlled-released cell growth factors and minimally invasive surgery. Int J Cardiol. 2004;95:S13-S15. Available from: https://doi.org/10.1016/S0167-5273(04)90004-6
- Kung JW, Currie IS, Forbes SJ, Ross JA. Liver development, regeneration, and carcinogenesis. J Biomed Biotechnol. 2010;2010:984248. Available from: https://doi.org/10.1155/2010/984248
Figures:
Similar Articles
Recently Viewed
-
Synthesis of Carbon Nano Fiber from Organic Waste and Activation of its Surface AreaHimanshu Narayan*,Brijesh Gaud,Amrita Singh,Sandesh Jaybhaye. Synthesis of Carbon Nano Fiber from Organic Waste and Activation of its Surface Area. Int J Phys Res Appl. 2019: doi: 10.29328/journal.ijpra.1001017; 2: 056-059
-
Obesity Surgery in SpainAniceto Baltasar*. Obesity Surgery in Spain. New Insights Obes Gene Beyond. 2020: doi: 10.29328/journal.niogb.1001013; 4: 013-021
-
Tamsulosin and Dementia in old age: Is there any relationship?Irami Araújo-Filho*,Rebecca Renata Lapenda do Monte,Karina de Andrade Vidal Costa,Amália Cinthia Meneses Rêgo. Tamsulosin and Dementia in old age: Is there any relationship?. J Neurosci Neurol Disord. 2019: doi: 10.29328/journal.jnnd.1001025; 3: 145-147
-
Case Report: Intussusception in an Infant with Respiratory Syncytial Virus (RSV) Infection and Post-Operative Wound DehiscenceLamin Makalo*,Orlianys Ruiz Perez,Benjamin Martin,Cherno S Jallow,Momodou Lamin Jobarteh,Alagie Baldeh,Abdul Malik Fye,Fatoumatta Jitteh,Isatou Bah. Case Report: Intussusception in an Infant with Respiratory Syncytial Virus (RSV) Infection and Post-Operative Wound Dehiscence. J Community Med Health Solut. 2025: doi: 10.29328/journal.jcmhs.1001051; 6: 001-004
-
The prevalence and risk factors of chronic kidney disease among type 2 diabetes mellitus follow-up patients at Debre Berhan Referral Hospital, Central EthiopiaGetaneh Baye Mulu,Worku Misganew Kebede,Fetene Nigussie Tarekegn,Abayneh Shewangzaw Engida,Migbaru Endawoke Tiruye,Mulat Mossie Menalu,Yalew Mossie,Wubshet Teshome,Bantalem Tilaye Atinafu*. The prevalence and risk factors of chronic kidney disease among type 2 diabetes mellitus follow-up patients at Debre Berhan Referral Hospital, Central Ethiopia. J Clini Nephrol. 2023: doi: 10.29328/journal.jcn.1001104; 7: 025-031
Most Viewed
-
Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth EnhancersH Pérez-Aguilar*, M Lacruz-Asaro, F Arán-Ais. Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth Enhancers. J Plant Sci Phytopathol. 2023 doi: 10.29328/journal.jpsp.1001104; 7: 042-047
-
Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case PresentationJulian A Purrinos*, Ramzi Younis. Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case Presentation. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001099; 8: 075-077
-
Feasibility study of magnetic sensing for detecting single-neuron action potentialsDenis Tonini,Kai Wu,Renata Saha,Jian-Ping Wang*. Feasibility study of magnetic sensing for detecting single-neuron action potentials. Ann Biomed Sci Eng. 2022 doi: 10.29328/journal.abse.1001018; 6: 019-029
-
Pediatric Dysgerminoma: Unveiling a Rare Ovarian TumorFaten Limaiem*, Khalil Saffar, Ahmed Halouani. Pediatric Dysgerminoma: Unveiling a Rare Ovarian Tumor. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001087; 8: 010-013
-
Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative reviewKhashayar Maroufi*. Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative review. J Sports Med Ther. 2021 doi: 10.29328/journal.jsmt.1001051; 6: 001-007

HSPI: We're glad you're here. Please click "create a new Query" if you are a new visitor to our website and need further information from us.
If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."