Abstract
Review Article
Neutrophils, NETs, NETosis and their paradoxical roles in COVID-19
Al-Anazi KA*, Al-Anazi WK and Al-Jasser AM
Published: 11 May, 2020 | Volume 4 - Issue 1 | Pages: 003-010
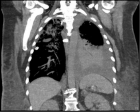
The pandemic of COVID-19 has adversely affected the world in many aspects. The health and economic sectors suffer most of the repercussions of this disease. The search for a cure for this rapidly spreading virus which is causing massive life losses worldwide requires clear understanding of the immunopathogenesis of this virus so as to develop pinpointed targeted therapies rather than relying mainly on supportive care measures and drug repurposing to fight this life-threatening virus infection.
Neutrophils, neutrophil extracellular traps (NETs), and NETosis are not well studied not only in COVID-19, but also in coroviruses in general. The review will shed lights on the functions of neutrophils, NETs, and NETosis in various infectious complications as well as in sepsis and acute lung conditions in an attempt to understand their actual roles and in order to help in designing targeted therapies in the near future.
Read Full Article HTML DOI: 10.29328/journal.jsctt.1001020 Cite this Article Read Full Article PDF
Keywords:
Neutrophils; Neutrophil extracellular traps; NETosis; COVID-19; Aute respiratory distress syndrome; Respiratory failure
References
- Wang L, Wang Y, Ye D, Liu Q. Review of the 2019 novel coronavirus (SARS-CoV-2) based on current evidence. Int J Antimicrob Agents. 2020; 105948. Pubmed: https://www.ncbi.nlm.nih.gov/pubmed/32201353
- Contini C, Di Nuzzo M, Barp N, Bonazza A, De Giorgio R, et al. The novel zoonotic COVID-19 pandemic: An expected global health concern. J Infect Dev Ctries. 2020; 14: 254-264. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/32235085
- Park SE. Epidemiology, virology, and clinical features of severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2; Coronavirus Disease-19). Clin Exp Pediatr. 2020; 63: 119-124. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/32252141
- Sohrabi C, Alsafi Z, O'Neill N, Khan M, Kerwan A, et al. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int J Surg. 2020; 76: 71-76. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/32112977
- Tu H, Tu S, Gao S, Shao A, Sheng J. The epidemiological and clinical features of COVID-19 and lessons from this global infectious public health event. J Infect. 2020; S0163-4453(20)30222-X. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/32315723
- Tay MZ, Poh CM, Rénia L, Mac Ary PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/32346093
- Yan Y, Shin WI, Pang YX, Meng Y, Lai J, et al. The first 75 days of novel coronavirus (SARS-CoV-2) outbreak: Recent advances, prevention, and treatment. Int J Environ Res Public Health. 2020; 17: E2323. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/32235575
- Sônego F, Castanheira FV, Ferreira RG, Kanashiro A, Leite CA, et al. Paradoxical roles of the neutrophil in sepsis: protective and deleterious. Front Immunol. 2016; 7: 155. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27199981
- Shiogama K, Onouchi T, Mizutani Y, Sakurai K, Inada K, et al. Visualization of neutrophil extracellular traps and fibrin meshwork in human fibrinopurulent inflammatory lesions: I. light microscopic study. Acta Histochem Cytochem. 2016; 49: 109-116. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27682014
- Hasler P, Giaglis S, Hahn S. Neutrophil extracellular traps in health and disease. Swiss Med Wkly. 2016; 146: w14352. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27723901
- Kaplan MJ, Radic M. Neutrophil extracellular traps: Double-edged swords of innate immunity. J Immunol. 2012; 189: 2689-2695. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22956760
- Knight JS, Carmona-Rivera C, Kaplan MJ. Proteins derived from neutrophil extracellular traps may serve as self-antigens and mediate organ damage in autoimmune diseases. Front Immunol. 2012; 3: 380. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23248629
- Bornhöfft KF, Viergutz T, Kühnle A, Galuska SP. Nanoparticles equipped with α2,8-linked sialic acid chains inhibit the release of neutrophil extracellular traps. Nanomaterials. 2019; 9: E610. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31013834
- Barrientos L, Marin-Esteban V, de Chaisemartin L, Le-Moal VL, Sandré C, et al. An improved strategy to recover large fragments of functional human neutrophil extracellular traps. Front Immunol. 2013; 4: 166. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23805143
- Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007; 176: 231-241. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17210947
- Chen L, Zhao Y, Lai D, Zhang P, Yang Y, et al. Neutrophil extracellular traps promote macrophage pyroptosis in sepsis. Cell Death Dis. 2018; 9: 597. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29789550
- Alasmari SZ. In vivo imaging of neutrophil extracellular traps (NETs): Visualization methods and outcomes. Biomed Res Int. 2020; 2020: 4192745. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/32090090
- Brinkmann V, Zychlinsky A. Neutrophil extracellular traps: Is immunity the second function of chromatin? J Cell Biol. 2012; 198: 773-783. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22945932
- Palmer LJ, Cooper PR, Ling MR, Wright HJ, Huissoon A, et al. Hypochlorous acid regulates neutrophil extracellular trap release in humans. Clin Exp Immunol. 2012; 167: 261-268. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22236002
- Cox LE, Walstein K, Völlger L, Reuner F, Bick A, et al. Neutrophil extracellular trap formation and nuclease activity in septic patients. BMC Anesthesiol. 2020; 20: 15. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31931719
- Barnes BJ, Adrover JM, Baxter-Stoltzfus A, Borczuk A, Cools-Lartigue J, et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J Exp Med. 2020; 217: e20200652. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/32302401
- Niedźwiedzka-Rystwej P, Repka W, Tokarz-Deptuła B, Deptuła W. In sickness and in health - how neutrophil extracellular trap (NET) works in infections, selected diseases and pregnancy. J Inflamm. 2019; 16: 15. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31297037
- Zhang F, Liu AL, Gao S, Ma S, Guo SB. Neutrophil dysfunction in sepsis. Chin Med J. 2016; 129: 2741-2744. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27824008
- Shen XF, Cao K, Jiang JP, Guan WX, Du JF. Neutrophil dysregulation during sepsis: an overview and update. J Cell Mol Med. 2017; 21: 1687-1697. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28244690
- Mortaz E, Alipoor SD, Adcock IM, Mumby S, Koenderman L. Update on neutrophil function in severe inflammation. Front Immunol. 2018; 9: 2171. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30356867
- Leliefeld PH, Wessels CM, Leenen LP, Koenderman L, Pillay J. The role of neutrophils in immune dysfunction during severe inflammation. Crit Care. 2016; 20: 73. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27005275
- Al-Jasser AM, Al-Anazi KA. Donor granulocyte transfusions in patients with hematologic malignancies and in recipients of hematopoietic stem cell transplantation. J Stem Cell Biol Transplant. 2019; 3: 1.
- Drewniak A, Kuijpers TW. Granulocyte transfusion therapy: randomization after all? Haematologica. 2009; 94: 1644-1648. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19996116
- Cui J, Wei X, Lv H, Li Y, Li P, et al. The clinical efficacy of intravenous IgM-enriched immunoglobulin (pentaglobin) in sepsis or septic shock: a meta-analysis with trial sequential analysis. Ann Intensive Care. 2019; 9: 27. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30725235
- Alejandria MM, Lansang MA, Dans LF, Mantaring JB 3rd. Intravenous immunoglobulin for treating sepsis, severe sepsis and septic shock. Cochrane Database Syst Rev. 2013; CD001090. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11869591
- Beyrau M, Bodkin JV, Nourshargh S. Neutrophil heterogeneity in health and disease: a revitalized avenue in inflammation and immunity. Open Biol. 2012; 2: 120134. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23226600
- Silvestre-Roig C, Fridlender ZG, Glogauer M, Scapini P. Neutrophil diversity in health and disease. Trends Immunol. 2019; 40: 565-583. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31160207
- Prame Kumar K, Nicholls AJ, Wong CHY. Partners in crime: neutrophils and monocytes/macrophages in inflammation and disease. Cell Tissue Res. 2018; 371: 551-565. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29387942
- Scapini P, Cassatella MA. Social networking of human neutrophils within the immune system. Blood. 2014; 124: 710-719. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24923297
- Kumar V, Sharma A. Neutrophils: Cinderella of innate immune system. Int Immunopharmacol. 2010; 10: 1325-1334. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20828640
- van Rees DJ, Szilagyi K, Kuijpers TW, Matlung HL, van den Berg TK. Immunoreceptors on neutrophils. Semin Immunol. 2016; 28: 94-108. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26976825
- Camp JV, Jonsson CB. A role for neutrophils in viral respiratory disease. Front Immunol. 2017; 8: 550. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28553293
- Galani IE, Andreakos E. Neutrophils in viral infections: Current concepts and caveats. J Leukoc Biol. 2015; 98: 557-564. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26160849
- Jenne CN, Wong CH, Zemp FJ, McDonald B, Rahman MM, et al. Neutrophils recruited to sites of infection protect from virus challenge by releasing neutrophil extracellular traps. Cell Host Microbe. 2013; 13: 169-180. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23414757
- D'Elia RV, Harrison K, Oyston PC, Lukaszewski RA, Clark GC. Targeting the cytokine storm for therapeutic benefit. Clin Vaccine Immunol. 2013; 20: 319-327. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23283640
- Bordon J, Aliberti S, Fernandez-Botran R, Uriarte SM, Rane MJ, et al. Understanding the roles of cytokines and neutrophil activity and neutrophil apoptosis in the protective versus deleterious inflammatory response in pneumonia. Int J Infect Dis. 2013; 17: e76-83. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23069683
- McDonald B, Kubes P. Chemokines: sirens of neutrophil recruitment-but is it just one song? Immunity. 2010; 33: 148-149. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20732637
- McDonald B, Urrutia R, Yipp BG, Jenne CN, Kubes P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe. 2012; 12: 324-333. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22980329
- Mozzini C, Girelli D. The role of neutrophil extracellular traps in COVID-19: Only an hypothesis or a potential new field. Thrombosis Res. 2020; 26-27. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/32360977
- Hiroki CH, Toller-Kawahisa JE, Fumagalli MJ, Colon DF, Figueiredo LTM, et al. Neutrophil extracellular traps effectively control acute Chikungunya virus infection. Front Immunol. 2020; 10: 3108. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/32082301
- Muraro SP, De Souza GF, Gallo SW, Da Silva BK, De Oliveira SD, et al. Respiratory syncytial virus induces the classical ROS-dependent NETosis through PAD-4 and necroptosis pathways activation. Sci Rep. 2018; 8: 14166. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30242250
- Schönrich G, Raftery MJ. Neutrophil extracellular traps go viral. Front Immunol. 2016; 7: 366. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27698656
- Opasawatchai A, Amornsupawat P, Jiravejchakul N, Chan-In W, Spoerk NJ, et al. Neutrophil activation and early features of NET formation are associated with Dengue virus infection in human. Front Immunol. 2019; 9: 3007. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30687301
- Schulz C, Gabriel G, von Köckritz-Blickwede M. Detrimental role of neutrophil extracellular traps during Dengue virus infection. Trends Immunol. 2020; 41: 3-6. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31791719
- Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010; 33: 657-670. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21094463
- Qin C, Zhou L, Hu Z, Zhang S, Yang S, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020. ciaa248. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/32161940
- Sun S, Cai X, Wang H, He G, Lin Y, et al. Abnormalities of peripheral blood system in patients with COVID-19 in Wenzhou, China. Clin Chim Acta. 2020. 507: 174-180. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/32339487
- Yang AP, Liu JP, Tao WQ, Li HM. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. 2020; 84: 106504. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/32304994
- Liu Y, Du X, Chen J, Jin Y, Peng L, et al. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect. 2020; S0163-4453(20)30208-5. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/32283162
- Zheng M, Gao Y, Wang G, Song G, Liu S, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020; 17: 533-535. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/32203188
- Ravindran M, Khan MA, Palaniyar N. Neutrophil extracellular trap formation: Physiology, pathology, and pharmacology. Biomolecules. 2019; 9: E365. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31416173
- Bystrzycka W, Moskalik A, Sieczkowska S, Manda-Handzlik A, Demkow U, et al. The effect of clindamycin and amoxicillin on neutrophil extracellular trap (NET) release. Cent Eur J Immunol. 2016; 41: 1-5. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27095915
- Onouchi T, Shiogama K, Mizutani Y, Takaki T, Tsutsumi Y. Visualization of neutrophil extracellular traps and fibrin meshwork in human fibrinopurulent inflammatory lesions: III. Correlative light and electron microscopic study. Acta Histochem Cytochem. 2016; 49: 141-147. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27917008
- Remijsen Q, Vanden Berghe T, Wirawan E, Asselbergh B, Parthoens E, et al. Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res. 2011; 21: 290-304. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21060338
- Neubert E, Meyer D, Kruss S, Erpenbeck L. The power from within - Understanding the driving forces of neutrophil extracellular trap formation. J Cell Sci. 2020; 133: jcs241075. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/32156720
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, et al. Neutrophil extracellular traps kill bacteria. Science. 2004; 303: 1532-1535. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15001782
- Vong L, Lorentz RJ, Assa A, Glogauer M, Sherman PM. Probiotic Lactobacillus rhamnosus inhibits the formation of neutrophil extracellular traps. J Immunol. 2014; 192: 1870-1877. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24465012
- Naffah de Souza C, Breda LCD, Khan MA, de Almeida SR, Câmara NOS, et al. Alkaline pH promotes NADPH oxidase-independent neutrophil extracellular trap formation: A matter of mitochondrial reactive oxygen species generation and citrullination and cleavage of histone. Front Immunol. 2018; 8: 1849. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29375550
- Steinberg BE, Grinstein S. Unconventional roles of the NADPH oxidase: Signaling, ion homeostasis, and cell death. Sci STKE. 2007; 2007: pe11. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17392241
- Hemmers S, Teijaro JR, Arandjelovic S, Mowen KA. PAD4-mediated neutrophil extracellular trap formation is not required for immunity against influenza infection. PLoS One. 2011; 6: e22043. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21779371
- Metzler KD, Fuchs TA, Nauseef WM, Reumaux D, Roesler J, et al. Myeloperoxidase is required for neutrophil extracellular trap formation: Implications for innate immunity. Blood. 2011; 117: 953-959. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20974672
- Chow OA, von Köckritz-Blickwede M, Bright AT, Hensler ME, Zinkernagel AS, et al. Statins enhance formation of phagocyte extracellular traps. Cell Host Microbe. 2010; 8: 445-454. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21075355
- Pallet N. Neutrophil extracellular traps orchestrate necroinflammation. J Am Soc Nephrol. 2017; 28: 1670-1672. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28232618
- Masuda S, Nakazawa D, Shida H, Miyoshi A, Kusunoki Y, et al. NETosis markers: Quest for specific, objective, and quantitative markers. Clin Chim Acta. 2016; 459: 89-93. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27259468
- Schneck E, Mallek F, Schiederich J, Kramer E, Markmann M, et al. Flow cytometry-based quantification of neutrophil extracellular traps shows an association with hypercoagulation in septic shock and hypocoagulation in postsurgical systemic inflammation-A proof-of-concept study. J Clin Med. 2020; 9: E174. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31936385
- de Buhr N, von Köckritz-Blickwede M. How neutrophil extracellular traps become visible. J Immunol Res. 2016; 2016: 4604713. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27294157
- Mohanty T, Sørensen OE, Nordenfelt P. NETQUANT: Automated quantification of neutrophil extracellular traps. Front Immunol. 2018; 8: 1999. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29379509
- Elsherif L, Sciaky N, Metts CA, Modasshir M, Rekleitis I, et al. Machine learning to quantitate neutrophil NETosis. Sci Rep. 2019; 9: 16891. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31729453
- Held P, Rumble J. Automated analysis of neutrophil NETosis activity using the Lionheart™ FX to image and analyze stimulated dHL60 cells. BioTek, Application Notes, Cellular Imaging, Live Cell Image. 2019.
- Boeltz S, Amini P, Anders HJ, Andrade F, Bilyy R, et al. To NET or not to NET: current opinions and state of the science regarding the formation of neutrophil extracellular traps. Cell Death Differ. 2019; 26: 395-408. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30622307
- Konig MF, Andrade F. A critical reappraisal of neutrophil extracellular traps and NETosis mimics based on differential requirements for protein citrullination. Front Immunol. 2016; 7: 461. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27867381
- Sørensen OE, Borregaard N. Neutrophil extracellular traps - the dark side of neutrophils. J Clin Invest. 2016; 126: 1612-1620. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27135878
- Bonaventura A, Vecchié A, Abbate A, Montecucco F. Neutrophil extracellular traps and cardiovascular diseases: An update. Cells. 2020; 9: E231. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31963447
- Kenny EF, Herzig A, Krüger R, Muth A, Mondal S, et al. Diverse stimuli engage different neutrophil extracellular trap pathways. Elife. 2017; 6: e24437. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28574339
- Twaddell SH, Baines KJ, Grainge C, Gibson PG. The emerging role of neutrophil extracellular traps in respiratory disease. Chest. 2019; 156: 774-782. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31265835
- Gan T, Yang Y, Hu F, Chen X, Zhou J, et al. TLR3 regulated poly I: C-induced neutrophil extracellular traps and acute lung injury partly through p38 MAP kinase. Front Microbiol. 2018; 9: 3174. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30622526
- Zhu L, Liu L, Zhang Y, Pu L, Liu J, et al. High level of neutrophil extracellular traps correlates with poor prognosis of severe influenza A infection. J Infect Dis. 2018; 217: 428-437. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29325098
- Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP, et al. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol. 2011; 179: 199-210. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21703402
- Hosseinzadeh A, Thompson PR, Segal BH, Urban CF. Nicotine induces neutrophil extracellular traps. J Leukoc Biol. 2016; 100: 1105-1112. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27312847
- Taghizadeh F, Akbari H. The powerful immune system against powerful COVID-19: A hypothesis. Med Hypothesis. 2020.
- Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: A review. Clin Immunol. 2020: 108427. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/32325252
- Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020. 138999. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/32329756
- Bendib I, de Chaisemartin L, Mekontso Dessap A, Chollet-Martin S, de Prost N. Understanding the role of neutrophil extracellular traps in patients with severe pneumonia and ARDS. Chest. 2019; 156: 1278-1280. Pubmed: https://www.ncbi.nlm.nih.gov/pubmed/31812204
- Geerdink RJ, Pillay J, Meyaard L, Bont L. Neutrophils in respiratory syncytial virus infection: A target for asthma prevention. J Allergy Clin Immunol. 2015; 136: 838-847. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26277597
- Domingo-Gonzalez R, Martínez-Colón GJ, Smith AJ, Smith CK, Ballinger MN, et al. Inhibition of neutrophil extracellular trap formation after stem cell transplant by prostaglandin E2. Am J Respir Crit Care Med. 2016; 193: 186-197. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26417909
- te Velthuis AJ, van den Worm SH, Sims AC, Baric RS, Snijder EJ, et al. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010; 6: e1001176. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21079686
Similar Articles
-
Neutrophils, NETs, NETosis and their paradoxical roles in COVID-19Al-Anazi KA*,Al-Anazi WK,Al-Jasser AM. Neutrophils, NETs, NETosis and their paradoxical roles in COVID-19. . 2020 doi: 10.29328/journal.jsctt.1001020; 4: 003-010
-
The rising role of mesenchymal stem cells in the treatment of COVID-19 infectionsAl-Anazi KA*,Al-Jasser AM. The rising role of mesenchymal stem cells in the treatment of COVID-19 infections. . 2020 doi: 10.29328/journal.jsctt.1001021; 4: 011-016
-
The complement cascade as a target against SARS-CoV-2-induced pneumoniaGianluigi Ferretti*. The complement cascade as a target against SARS-CoV-2-induced pneumonia. . 2023 doi: 10.29328/journal.jsctt.1001029; 7: 001-002
-
Update on the Use of Mesenchymal Stem Cells in the Treatment of Various Infectious Diseases Including COVID-19 InfectionKhalid A Al-Anazi*, Rehab Y Al-Ansari. Update on the Use of Mesenchymal Stem Cells in the Treatment of Various Infectious Diseases Including COVID-19 Infection. . 2023 doi: 10.29328/journal.jsctt.1001033; 7: 034-042
Recently Viewed
-
Atypical Anti-GBM with ANCA Vasculitis- A Rarest of the Rare Entity: Index Case from Eastern IndiaGopambuj Singh Rathod*, Atanu Pal, Pallavi Mahato, Aakash Roy, Debroop Sengupta, Muzzamil Ahmad. Atypical Anti-GBM with ANCA Vasculitis- A Rarest of the Rare Entity: Index Case from Eastern India. J Clini Nephrol. 2024: doi: 10.29328/journal.jcn.1001139; 8: 124-126
-
Gallstone Ileus: A Rare Case of Intestinal Obstruction, Presented in a Chronic Kidney Disease Patient on HaemodialysisPulak Azad*,Yasir Sultan Rizvi,Lakshmi Kant Jha,Pranav Tyagi,Sachin Jain,Twinkle Malik. Gallstone Ileus: A Rare Case of Intestinal Obstruction, Presented in a Chronic Kidney Disease Patient on Haemodialysis. J Clini Nephrol. 2025: doi: 10.29328/journal.jcn.1001149; 9: 027-030
-
A Case of Rapidly Progressive Renal Failure with Unearthed AmyloidosisPulak Azad*,Lakshmi Kant Jha,Yasir Sultan Rizvi,Pranav Tyagi. A Case of Rapidly Progressive Renal Failure with Unearthed Amyloidosis. J Clini Nephrol. 2025: doi: 10.29328/journal.jcn.1001140; 9: 024-026
-
An Adult Case of Beta Thalassemia with Right Ventricular Outflow Tract Tachycardia: A Case ReportPrem AS,Shahanas PS,Praveen Sreekumar*,Ramaswamy NV. An Adult Case of Beta Thalassemia with Right Ventricular Outflow Tract Tachycardia: A Case Report. J Cardiol Cardiovasc Med. 2024: doi: 10.29328/journal.jccm.1001201; 9: 177-179
-
Autoantibodies in Autoimmune Addison’s Disease: Why are they Important?Maria Rosaria De Cagna, Norma Notaristefano, Maurizio Schiavone, Gianluca Palatella, Federica Ranù, Carmela Presicci, Valerio Cecinati, Marilina Tampoia*. Autoantibodies in Autoimmune Addison’s Disease: Why are they Important?. Arch Pathol Clin Res. 2024: doi: 10.29328/journal.apcr.1001042; 8: 012-015
Most Viewed
-
Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth EnhancersH Pérez-Aguilar*, M Lacruz-Asaro, F Arán-Ais. Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth Enhancers. J Plant Sci Phytopathol. 2023 doi: 10.29328/journal.jpsp.1001104; 7: 042-047
-
Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case PresentationJulian A Purrinos*, Ramzi Younis. Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case Presentation. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001099; 8: 075-077
-
Feasibility study of magnetic sensing for detecting single-neuron action potentialsDenis Tonini,Kai Wu,Renata Saha,Jian-Ping Wang*. Feasibility study of magnetic sensing for detecting single-neuron action potentials. Ann Biomed Sci Eng. 2022 doi: 10.29328/journal.abse.1001018; 6: 019-029
-
Pediatric Dysgerminoma: Unveiling a Rare Ovarian TumorFaten Limaiem*, Khalil Saffar, Ahmed Halouani. Pediatric Dysgerminoma: Unveiling a Rare Ovarian Tumor. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001087; 8: 010-013
-
Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative reviewKhashayar Maroufi*. Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative review. J Sports Med Ther. 2021 doi: 10.29328/journal.jsmt.1001051; 6: 001-007

HSPI: We're glad you're here. Please click "create a new Query" if you are a new visitor to our website and need further information from us.
If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."